Thermodynamic coupling of reactions via few-molecule vibrational polaritons
Abstract
Interaction between light and molecular vibrations leads to hybrid light-matter states called vibrational polaritons. Even though many intriguing phenomena have been predicted for single-molecule vibrational strong coupling (VSC), several studies suggest that these effects tend to be diminished in the many-molecule regime due to the presence of dark states. Achieving single or few-molecule vibrational polaritons has been constrained by the need for fabricating extremely small mode volume infrared cavities. In this work, we propose an alternative strategy to achieve
single-molecule VSC in a cavity-enhanced Raman spectroscopy (CERS) setup, based on the physics of cavity optomechanics. We then present a scheme harnessing few-molecule VSC to thermodynamically couple two reactions, such that a spontaneous electron transfer can now fuel a thermodynamically uphill reaction that was non-spontaneous outside the cavity.
Keywords. Few-molecule polaritons, cavity-enhanced Raman spectroscopy, cavity-optomechanics, coupled chemical reactions, thermodynamic driving.
Introduction
Strong coupling (SC) ensues when the rate of coherent energy exchange between matter degrees of freedom (DOF) and a confined electromagnetic field exceeds the losses from either of them [1, 2, 3, 4]. This interplay leads to the emergence of hybrid light-matter states called polaritons [5, 6, 7], which inherit properties from both the photonic and the matter constituents. For molecular systems, due to the small magnitude of the transition dipole moment of most individual molecules, SC is typically achieved by having an ensemble of molecules interact with a cavity mode [5, 8]. In this collective case, in addition to two polariton states, SC leads to () dark states which are predominantly molecular in character [9]. In both the electronic and vibrational regimes, harnessing these hybrid light-matter states has led to the emergence of a plethora of polariton-based devices [10] such as amplifiers [11, 12], tunneling diodes [13], routers [14], and ultrafast switches [15, 16, 17]; and novel phenomena like enhanced energy and charge transport [18, 19, 20], modification and control of a chemical reaction without external pumping [21, 22], and remote catalysis [23].
Theoretical models of polaritons often use a single molecule with a collective superradiant coupling to the cavity to explain the experimentally observed effects of collective SC on physical and chemical phenomena [24, 25, 26, 27]. However, several theoretical studies, that account for the large number of molecules coupled to the cavity, suggest that SC could be rendered less effective in the collective regime owing to the entropic penalty from the dark states [6, 28]. For enhanced polaritonic effects, the state-of-the-art is either to use polariton condensates [29, 30] or to achieve single-molecule SC[31]. In the electronic regime, both polariton condensation [32, 33, 34, 35] and single-molecule SC [31, 36] have been achieved.
There have been theoretical proposals of ways to achieve a vibrational polariton condensate [37]. However, to the best of our knowledge, in the vibrational regime, neither condensation nor single-molecule SC has yet been experimentally demonstrated. The bottleneck for single molecule SC in the vibrational case is the fabrication of low-mode volume cavities in the infrared (IR) regime [38]. This calls for alternate strategies to attain vibrational SC with a single or few molecules.
In this work, we propose using optomechanics as a way to achieve SC for molecular vibrations. Over the last decade, optomechanics has emerged as a powerful tool in quantum technologies with applications [39] such as backaction cooling of a mechanical oscillator [40, 41], parametric amplification [42, 43], optomechanically induced transparency [44, 45], and generation of non-classical quantum states [46, 47]. Aspelmeyer and co-workers have demonstrated SC in an optomechanical architecture, for a micromechanical resonator coupled to an optical cavity setup [48]. It has been shown recently that surface-enhanced and cavity-enhanced Raman spectroscopy (SERS/CERS) can be understood through the theoretical framework of cavity optomechanics [49, 50, 51, 52, 53, 54]. Here we exploit this observation to demonstrate that a single molecule in a CERS setup, under strong illumination of a red-detuned laser can be a viable platform to achieve the long-standing goal of single and few-molecule vibrational polaritons. Few-molecule polaritons do not suffer from the deleterious effects of a macroscopic number of dark states, and hence are better candidates for harnessing the properties of polaritons [5].
As a proof-of-concept application of few-molecule vibrational polaritons, we will introduce the intriguing concept of coupling chemical reactions via the latter. Biological systems use coupled chemical reactions and thermodynamics to their advantage by driving energetically uphill processes, such as active transport, using spontaneous reactions, like the dissociation of ATP [55]. Humans have looked towards nature for inspiration and translated biological knowledge into innovative products and processes [56, 57]. We shall show how the delocalization of the polariton modes inside the cavity can be exploited to design a biomimetic of ATP-driven molecular machines.
Results and Discussion
Model

Our theoretical model considers a single molecule placed inside a UV-vis cavity, such that the cavity frequency is off-resonant to any optically allowed transitions of the molecule. In this regime, the coupling between the cavity and the molecule is purely parametric through the molecule’s polarizability, with the vibration of the molecule causing a dispersive shift in the cavity resonance [49]. Due to better spatial overlap between the mode profile of the cavity and the molecule, we consider using a Fabry-Perót cavity. However, the formalism presented here is valid for other cavity types. We show that the cavity-molecule system, when pumped with a laser that is red-detuned from the cavity and the detuning is in the order of the molecule’s vibrational frequency
(Figure 1b), yields an effective Hamiltonian resembling the vibrational polaritonic Hamiltonian. Importantly, the light-matter coupling can be tuned by varying the laser power.
We model the photon mode and the vibration of the molecule as harmonic oscillators with frequencies and , and annihilation operators and , respectively. The cavity has a decay rate of and the vibrational mode has a decay rate of . In this work, the losses will be modeled using Lindblad master equations [58]. Since the polarizability, , of the molecule, to the leading order, depends on its vibrational displacement [53], , the Hamiltonian for the cavity-molecule system is [49]
| (1) |
where is the vacuum cavity-molecule coupling, with and as the mode volume of the cavity and vacuum permittivity, respectively. This Hamiltonian formally resembles an optomechanical setup [49], where the displacement of a mechanical oscillator modulates the frequency of the cavity. The cavity then acts back on the oscillator through radiation pressure force, which is a function of the cavity’s photon occupation.
We drive the cavity with a laser red-detuned , from the cavity resonance. The full Hamiltonian for a laser mode with annihilation operator coupled to the cavity-molecule subsystem is given as
| (2) |
with the cavity-laser coupling . Here, is related to the laser power with being the mean photon number in the laser mode [59].
We make the rotating wave approximation (RWA) in the laser-cavity coupling and diagonalize the laser-cavity subsystem. The operators and represent the new ‘laser-like’ and ‘cavity-like’ normal modes of the laser-cavity subsystem with frequencies and , respectively. The Hamiltonian after making the approximation and change of basis is
| (3) | |||||
where is the laser-cavity mixing angle [60].
Considering the red-detuned case for the cavity-laser detuning, , in the order of , we drop the off-resonant contributions in the cavity-molecule interaction term, simplifying to
| (4) | |||||
We will later set the laser-cavity detuning .
We define a composite laser-cavity photon mode with annihilation operator (Figure 1b), where and are the mean photon occupations in the ‘laser-like’ and ‘cavity-like’ normal modes, respectively. The Heisenberg equations of motion for the operators and in the mean-field approximation [61],
| (5a) | ||||
| (5b) | ||||
yield the effective Hamiltonian
| (6) | |||||
when and (see Supplementary note-1). Here we have suggestively defined . For an input laser drive with power , the interaction strength between the composite photon and the molecular vibration transforms to , consistent with the results obtained from the classical treatments of the laser mode [54, 52, 39, 59].
When , resembles a vibrational polaritonic Hamiltonian, where the composite photon mode is resonant with the vibrational DOF (Figure 1b) [5]. Here the coupling strength is tunable by changing the pumping power of the laser. This can, in principle, foster the SC regime when the coupling strength supersedes the decay processes in the system. To look at parameter sets yielding this regime and to compute spectra, we simulate the dynamics of the density matrix () of the system using Lindblad master equations [53, 58] given as
| (7) |
The last four terms on the right-hand side are the Lindblad-Kossakowski terms defined as . Here, models the incoherent decay from the composite photon mode. The incoherent decay, thermal pumping, and pure dephasing of the vibrational mode by the environment at temperature are modeled by
the , , and terms, respectively, where is the Bose-Einstein distribution function at transition energy . Additionally, in the limit of large photon number in the laser (), assuming the photon occupation to be constant, and thus the laser mode to be non-lossy, the decay rate of the composite photon equals the cavity decay rate (see Supplementary note-2).
The simulations have been performed using the QuTip package [62, 63] and the results are presented in Figure 2 for the molecule Rhodamine 6G [49], where eV, ( eV), ( eV), ( eV), ( eV). Here, ( eV) and are the rates for vibrational relaxation and pure dephasing, respectively [6] . The fluence of the lasers is chosen to be below MW [49, 64] with a beam area of m2. Figure 2a shows the effective light-matter coupling, , as a function of laser fluence, , and single photon coupling strength, . In Figure 2b, the vibrational spectrum of the molecule , splits, demonstrating SC. We see the Rabi-splitting increases with laser power, thus giving us additional control over the light-matter coupling strength. Figure 2c shows the spectra with one, two, and four molecules for constant laser power. Figure 2d is the vibrational spectrum of the molecule as a function of the cavity-laser detuning. The avoided crossing at the detuning () equal to the vibrational frequency () demonstrates maximal hybridization between the photonic and matter DOF. Finally, Figure 2e and f show the emission spectra from the cavity at steady state (ss), [65, 53, 66], also revealing the polariton peaks.

Polariton-assisted thermodynamic driving
The matter component of the polariton modes is delocalized over many molecules under collective SC [67, 5]. This delocalization can be exploited more effectively with few-molecule polaritons, owing to reduced involvement of dark modes [31, 9], which remain parked essentially at the same energy as the original molecular transitions. In this work, we consider the molecular species undergoing electron-transfer reactions, modeled using Marcus-Levich-Jortner (MLJ) theory [68, 69, 70]. Our system consists of two reactive molecules A and B of different species placed inside an optomechanical cavity. Here molecule A features a spontaneous reaction (with negative free energy change, ), while molecule B features an endergonic reaction (, with ). We demonstrate thermodynamic coupling between the two molecular species via the composite photon mode, such that the spontaneous electron transfer in A can drive B to react. Schematically, electron transfer in A creates a vibrationally hot product (Figure 3a), which, outside the cavity, just decays to the product ground state. However, inside the cavity, in the timescale of the Rabi frequency, this excitation can be captured by the photon mode, which then can excite the reactant in B to its vibrational excited state. The electron transfer in B can then proceed spontaneously from the reactant’s excited state (Figure 3b). Notably, this scheme can also be generalized to other types of reactions.

Within the framework of MLJ theory, the molecules can exist in either of the two diabatic electronic states: corresponding to the reactant and corresponding to the product for molecule (Figure 3a). For molecule B, in this case the switching between and through electron transfer contributes to useful mechanical work, manifested in changes of nuclear configuration [71, 72, 73]. The electronic states for each molecule are dressed with a local high-frequency intramolecular vibrational coordinate represented by annihilation operator , for , and coupled to a low-frequency effective solvent mode treated classically with rescaled momentum and position as and . respectively. We assume that the high-frequency modes of both species, being resonant, are the only ones that couple to the composite photon [74]. Upon reaction, the high-frequency mode undergoes a change in its equilibrium configuration according to, , where is the displacement operator, and is the Huang-Rhys factor [37]. The Hamiltonian describing the system is given as , where
| (8a) | ||||
| (8b) | ||||
Here is the bare Hamiltonian corresponding to the composite photon mode consisting of the laser and the cavity, represents the high-frequency mode and the solvent mode associated with molecule ,
| (9a) | ||||
| (9b) | ||||
with and being the displacement and frequency along the solvent coordinate, respectively, and , the free energy difference for the molecular species . Additionally, is the effective coupling between the photonic and molecular DOF. For simplicity, we assume that the reaction involves a vibrational mode with nearly identical frequency and light-matter coupling strength for species A and B in both the reactant and product electronic states ( and ). Finally, the diabatic couplings between the electronic states and are given by , where is the coupling strength.
We can solve parametrically as a function of the solvent coordinates to construct the potential energy surfaces(PES) (Figure 4a and b). Considering these diabatic couplings to be perturbative, can be diagonalized to obtain the two polariton modes, , with frequencies , and one dark mode, , with frequency , given as
| (10a) | |||||
| (10b) | |||||
such that, and . Here, is the mixing angle, where is the detuning between the composite photon mode and the molecule. Here we have chosen the composite photon mode to be resonant with the intramolecular vibration, i.e., .
We now define multi-particle states that span the Hilbert space of the system, where , corresponds to the electronic DOF, and to the cavity-vibrational mode of each electronic state [6]. To describe the reaction, we look at the population dynamics in the electronic states. The kinetic master equations governing the time evolution of the system are given as [6]
| (11) |
where represents the population in state, and is the rate constant for population transfer from to due to processes like reactive transitions between the electronic states accompanied by solvent reorganization and decay through the cavity and vibrational DOF. The rate constant for the reactive transition at a temperature within the framework of MLJ theory is given as [74]
| (12) |
Here, is the energy of the state , and and are the solvent reorganization energy and diabatic coupling, respectively, corresponding to the reacting species. Additionally, represent the Franck-Condon factors for the hybrid photon-vibration states and corresponding to the electronic states and , respectively. For the simulations in this work, the Franck-Condon factors have been computed numerically from eigenstates obtained using
the standard discrete-variable representation (DVR) of Colbert and Miller [75].
The reactive transitions transfer populations across different electronic states, while the cavity and vibrational decays lead to dynamics within the same electronic state. In these simulations, since , we restrict ourselves to the first excitation manifold in the photon-vibration DOF. With the bare vibrational decay rate for the intramolecular vibrations for molecule A and B as and , respectively, and bare cavity decay rate as , we have
| (13) |
where represents a single excitation in the polaritons ) or the dark () mode, and the ’s correspond to the expansion coefficients of the excited eigenmode in terms of the cavity and vibrational modes [76].
Finally, the anharmonic couplings between the vibrational mode of interest and an other bath of low frequency modes leads to transitions between the polaritons and the dark mode, [6]
| (14) |
where is the heavyside step function, is the Bose-Einstein distribution function at the transition energy , and is the spectral density of the low frequency modes. Assuming the spectral density to be Ohmic [77], we have , where is a dimensionless parameter modeling the anharmonic system–bath interactions and is the cut-off frequency for the low-frequency modes.
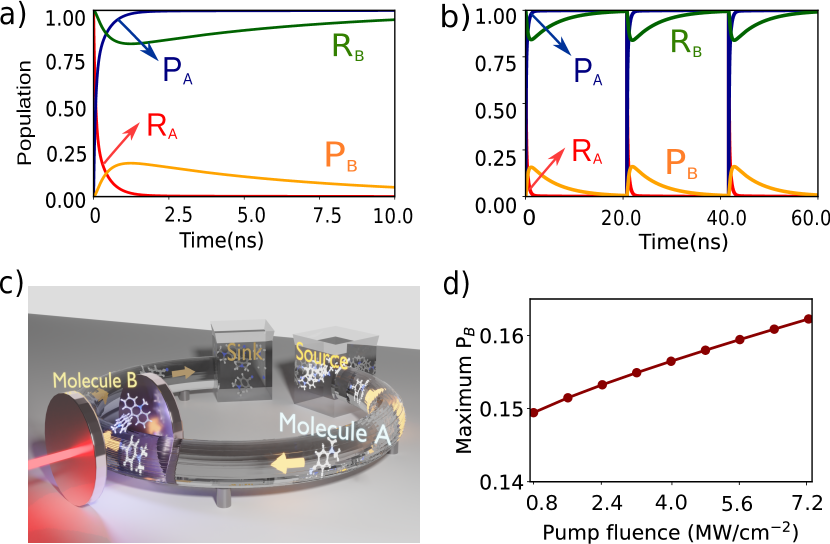
The results of the simulations are presented in Figure 4. Here, eV (call ), ( eV), MW/cm2, A=5 m2, ( eV) [6], eV, ( eV), ( eV), ( eV), ( eV), ( eV), ( eV), and [6]. The decay rates have been chosen to be similar to those typically found in VSC experiments [78, 61, 79]. The diabatic couplings are chosen to be eV) and K. We start from the initial electronic state . Independently, the reaction of molecule A is spontaneous due to its negative free energy change, , while molecule B remains in its thermodynamically stable conformer (Figure 4a and b). This reflects the dynamics of the species outside of the cavity. Placing both the molecules inside the cavity couples the two reactions via the photonic mode enabling the spontaneity of the reaction of molecule A to thermodynamically ‘lift’ B to its unstable configuration producing mechanical work. However, after molecule A has fully reacted (change in nuclear configuration), inevitably B has to relax again to its stable configuration , completing one cycle of the mechanical motion of B (Figure 3b). The maximum population obtained in before molecule B relaxes back to increases with the light-matter coupling strength (), tunable with the fluence of the driving laser (Figure 4d). For the cycle to be repeated, molecule A needs to be ‘recharged’ or ‘replaced’. To achieve this, we envision a flow setup, as schematically depicted in Figure 4c, that can circulate molecule A inside and out of the cavity. Continued circulation of the A molecules is essential for the molecular machine of B to be oscillating between reactant and product and producing mechanical work (Figure 4a and b). This phenomenon realizes a heat engine producing mechanical work in molecule B, using the (chemical) energy flow from a ‘source’ to a ‘sink’ bath in the form of molecule A [80].
Conclusion
We have shown that the physics of cavity optomechanics can be harnessed in CERS to achieve single to few-molecule vibrational SC using laser-driven UV-vis cavities. We show that the coupling strength and hence the Rabi splitting is tunable with the laser intensity, and it is achievable with realistic pump powers and cavity-molecule couplings. SC in the few molecules regime can avail enhanced polaritonic effects owing to the reduced entropic penalty from the dark states. By using the MLJ theory for electron transfer, we show that the photon-mediated coupling between two reactions, one spontaneous and one non-spontaneous, can be exploited to thermodynamically drive the non-spontaneous process using the spontaneous one. This effect is analogous to harnessing ATP to drive uphill biological processes like the active transport of ions across a membrane against their concentration gradient and can be used to design bio-inspired molecular machines [81]. Moving forward, an experimental realization of the scheme for vibrational SC presented here would be a significant step towards utilizing polaritons for chemistry.
Code availability
Computational scripts used to generate the plots in the present article are available by email upon request to the authors.
Acknowledgements.
This work was supported as part of the Center for Molecular Quantum Transduction (CMQT), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under Award No. DE-SC0021314. A.K. thanks Yong Rui Poh, Kai Schwenickke, Juan B. Pérez-Sánchez, Alex Fairhall, and Carlos A. Saavedra Salazar for useful discussions.References
- Kavokin et al. [2017] A. Kavokin, J. Baumberg, G. Malpuech, and F. Laussy, Microcavities, Series on Semiconductor Science and Technology (OUP Oxford, 2017).
- Törmä and Barnes [2014] P. Törmä and W. L. Barnes, Reports on Progress in Physics 78, 013901 (2014).
- Bitton et al. [2019] O. Bitton, S. N. Gupta, and G. Haran, Nanophotonics 8, 559 (2019).
- Bourgeois et al. [2022] M. R. Bourgeois, E. K. Beutler, S. Khorasani, N. Panek, and D. J. Masiello, Phys. Rev. Lett. 128, 197401 (2022).
- Ribeiro et al. [2018] R. F. Ribeiro, L. A. Martínez-Martínez, M. Du, J. Campos-Gonzalez-Angulo, and J. Yuen-Zhou, Chem. Sci. 9, 6325 (2018).
- Du et al. [2021] M. Du, J. A. Campos-Gonzalez-Angulo, and J. Yuen-Zhou, The Journal of Chemical Physics 154, 084108 (2021).
- Dunkelberger et al. [2022a] A. D. Dunkelberger, B. S. Simpkins, I. Vurgaftman, and J. C. Owrutsky, Annual Review of Physical Chemistry 73, 429 (2022a).
- Hirai et al. [2020a] K. Hirai, J. A. Hutchison, and H. Uji-i, ChemPlusChem 85, 1981 (2020a).
- Du and Yuen-Zhou [2022] M. Du and J. Yuen-Zhou, Phys. Rev. Lett. 128, 096001 (2022).
- Fraser [2017] M. D. Fraser, Semiconductor Science and Technology 32, 093003 (2017).
- Berini and De Leon [2012] P. Berini and I. De Leon, Nature Photonics 6, 16 (2012).
- Jamadi et al. [2018] O. Jamadi, F. Reveret, P. Disseix, F. Medard, J. Leymarie, A. Moreau, D. Solnyshkov, C. Deparis, M. Leroux, E. Cambril, S. Bouchoule, J. Zuniga-Perez, and G. Malpuech, Light: Science & Applications 7, 82 (2018).
- Nguyen et al. [2013] H. S. Nguyen, D. Vishnevsky, C. Sturm, D. Tanese, D. Solnyshkov, E. Galopin, A. Lemaître, I. Sagnes, A. Amo, G. Malpuech, and J. Bloch, Phys. Rev. Lett. 110, 236601 (2013).
- Marsault et al. [2015] F. Marsault, H. S. Nguyen, D. Tanese, A. Lemaître, E. Galopin, I. Sagnes, A. Amo, and J. Bloch, Applied Physics Letters 107, 201115 (2015).
- Amo et al. [2010] A. Amo, T. C. H. Liew, C. Adrados, R. Houdré, E. Giacobino, A. V. Kavokin, and A. Bramati, Nature Photonics 4, 361 (2010).
- Chen et al. [2022] F. Chen, H. Li, H. Zhou, S. Luo, Z. Sun, Z. Ye, F. Sun, J. Wang, Y. Zheng, X. Chen, H. Xu, H. Xu, T. Byrnes, Z. Chen, and J. Wu, Phys. Rev. Lett. 129, 057402 (2022).
- Pscherer et al. [2021] A. Pscherer, M. Meierhofer, D. Wang, H. Kelkar, D. Martín-Cano, T. Utikal, S. Götzinger, and V. Sandoghdar, Phys. Rev. Lett. 127, 133603 (2021).
- Wang et al. [2021] M. Wang, M. Hertzog, and K. Börjesson, Nature Communications 12, 1874 (2021).
- Du et al. [2018] M. Du, L. A. Martínez-Martínez, R. F. Ribeiro, Z. Hu, V. M. Menon, and J. Yuen-Zhou, Chem. Sci. 9, 6659 (2018).
- Groenhof and Toppari [2018] G. Groenhof and J. J. Toppari, The Journal of Physical Chemistry Letters 9, 4848 (2018).
- Thomas et al. [2019] A. Thomas, L. Lethuillier-Karl, K. Nagarajan, R. M. A. Vergauwe, J. George, T. Chervy, A. Shalabney, E. Devaux, C. Genet, J. Moran, and T. W. Ebbesen, Science 363, 615 (2019).
- Hirai et al. [2020b] K. Hirai, R. Takeda, J. A. Hutchison, and H. Uji-i, Angewandte Chemie International Edition 59, 5332 (2020b).
- Du et al. [2019] M. Du, R. F. Ribero, and J. Yuen-Zhou, Chem 5, 1167 (2019).
- Haugland et al. [2020] T. S. Haugland, E. Ronca, E. F. Kjønstad, A. Rubio, and H. Koch, Phys. Rev. X 10, 041043 (2020).
- Sidler et al. [2021] D. Sidler, C. Schäfer, M. Ruggenthaler, and A. Rubio, The Journal of Physical Chemistry Letters 12, 508 (2021).
- Basov et al. [2021] D. N. Basov, A. Asenjo-Garcia, P. J. Schuck, X. Zhu, and A. Rubio, Nanophotonics 10, 549 (2021).
- May et al. [2020] M. A. May, D. Fialkow, T. Wu, K.-D. Park, H. Leng, J. A. Kropp, T. Gougousi, P. Lalanne, M. Pelton, and M. B. Raschke, Advanced Quantum Technologies 3, 1900087 (2020).
- Li et al. [2022] T. E. Li, B. Cui, J. E. Subotnik, and A. Nitzan, Annual Review of Physical Chemistry 73, 43 (2022).
- Keeling and Kéna-Cohen [2020] J. Keeling and S. Kéna-Cohen, Annual Review of Physical Chemistry 71, 435 (2020).
- Cortese et al. [2017] E. Cortese, P. G. Lagoudakis, and S. De Liberato, Phys. Rev. Lett. 119, 043604 (2017).
- Chikkaraddy et al. [2016] R. Chikkaraddy, B. de Nijs, F. Benz, S. J. Barrow, O. A. Scherman, E. Rosta, A. Demetriadou, P. Fox, O. Hess, and J. J. Baumberg, Nature 535, 127 (2016).
- Zasedatelev et al. [2019] A. V. Zasedatelev, A. V. Baranikov, D. Urbonas, F. Scafirimuto, U. Scherf, T. Stöferle, R. F. Mahrt, and P. G. Lagoudakis, Nature Photonics 13, 378 (2019).
- Kéna-Cohen and Forrest [2010] S. Kéna-Cohen and S. R. Forrest, Nature Photonics 4, 371 (2010).
- Baumberg et al. [2008] J. J. Baumberg, A. V. Kavokin, S. Christopoulos, A. J. D. Grundy, R. Butté, G. Christmann, D. D. Solnyshkov, G. Malpuech, G. Baldassarri Höger von Högersthal, E. Feltin, J.-F. Carlin, and N. Grandjean, Phys. Rev. Lett. 101, 136409 (2008).
- Ghosh and Liew [2020] S. Ghosh and T. C. H. Liew, npj Quantum Information 6, 16 (2020).
- Pelton et al. [2019] M. Pelton, S. D. Storm, and H. Leng, Nanoscale 11, 14540 (2019).
- Pannir-Sivajothi et al. [2022] S. Pannir-Sivajothi, J. A. Campos-Gonzalez-Angulo, L. A. Martínez-Martínez, S. Sinha, and J. Yuen-Zhou, Nature Communications 13, 1645 (2022).
- Mondal et al. [2022] M. Mondal, A. Semenov, M. A. Ochoa, and A. Nitzan, The Journal of Physical Chemistry Letters 13, 9673 (2022).
- Aspelmeyer et al. [2014] M. Aspelmeyer, T. J. Kippenberg, and F. Marquardt, Rev. Mod. Phys. 86, 1391 (2014).
- Elste et al. [2009] F. Elste, S. M. Girvin, and A. A. Clerk, Phys. Rev. Lett. 102, 207209 (2009).
- Metzger and Karrai [2004] C. H. Metzger and K. Karrai, Nature 432, 1002 (2004).
- Massel et al. [2011] F. Massel, T. T. Heikkilä, J.-M. Pirkkalainen, S. U. Cho, H. Saloniemi, P. J. Hakonen, and M. A. Sillanpää, Nature 480, 351 (2011).
- Clerk et al. [2010] A. A. Clerk, M. H. Devoret, S. M. Girvin, F. Marquardt, and R. J. Schoelkopf, Rev. Mod. Phys. 82, 1155 (2010).
- Fleischhauer et al. [2005] M. Fleischhauer, A. Imamoglu, and J. P. Marangos, Rev. Mod. Phys. 77, 633 (2005).
- Safavi-Naeini et al. [2011] A. H. Safavi-Naeini, T. P. M. Alegre, J. Chan, M. Eichenfield, M. Winger, Q. Lin, J. T. Hill, D. E. Chang, and O. Painter, Nature 472, 69 (2011).
- Rashid et al. [2016] M. Rashid, T. Tufarelli, J. Bateman, J. Vovrosh, D. Hempston, M. S. Kim, and H. Ulbricht, Phys. Rev. Lett. 117, 273601 (2016).
- Tan et al. [2013] H. Tan, G. Li, and P. Meystre, Phys. Rev. A 87, 033829 (2013).
- Gröblacher et al. [2009] S. Gröblacher, K. Hammerer, M. R. Vanner, and M. Aspelmeyer, Nature 460, 724 (2009).
- Roelli et al. [2016] P. Roelli, C. Galland, N. Piro, and T. J. Kippenberg, Nature Nanotechnology 11, 164 (2016).
- Schmidt et al. [2017] M. K. Schmidt, R. Esteban, F. Benz, J. J. Baumberg, and J. Aizpurua, Faraday Discuss. 205, 31 (2017).
- Zhang et al. [2020] Y. Zhang, J. Aizpurua, and R. Esteban, ACS Photonics 7, 1676 (2020).
- Neuman et al. [2019] T. Neuman, R. Esteban, G. Giedke, M. K. Schmidt, and J. Aizpurua, Phys. Rev. A 100, 043422 (2019).
- Schmidt et al. [2016] M. K. Schmidt, R. Esteban, A. González-Tudela, G. Giedke, and J. Aizpurua, ACS Nano 10, 6291 (2016).
- Esteban et al. [2022] R. Esteban, J. J. Baumberg, and J. Aizpurua, Accounts of Chemical Research 55, 1889 (2022).
- Tinoco et al. [2013] I. Tinoco, K. Sauer, J. Wang, J. Puglisi, G. Harbison, and D. Rovnyak, Physical Chemistry: Principles and Applications in Biological Sciences (Pearson Education, 2013).
- Breslow [2009] R. Breslow, Journal of Biological Chemistry 284, 1337 (2009).
- Hong et al. [2010] Y. Hong, D. Velegol, N. Chaturvedi, and A. Sen, Physical Chemistry Chemical Physics 12, 1423 (2010).
- Nitzan [2013] A. Nitzan, Chemical dynamics in condensed phases: Relaxation, transfer, and reactions in condensed molecular systems (OUP Oxford, 2013) Chap. 10.
- Steck [2007] D. Steck, Quantum and atom optics (2007) Chap. 12, pp. 509–518.
- Mandal et al. [2020] A. Mandal, T. D. Krauss, and P. Huo, The Journal of Physical Chemistry B 124, 6321 (2020).
- F. Ribeiro et al. [2018] R. F. Ribeiro, A. D. Dunkelberger, B. Xiang, W. Xiong, B. S. Simpkins, J. C. Owrutsky, and J. Yuen-Zhou, The Journal of Physical Chemistry Letters 9, 3766 (2018).
- Johansson et al. [2012a] J. Johansson, P. Nation, and F. Nori, Computer Physics Communications 183, 1760 (2012a).
- Johansson et al. [2012b] J. Johansson, P. Nation, and F. Nori, Computer Physics Communications 183, 1760 (2012b).
- Werner and Hashimoto [2011] D. Werner and S. Hashimoto, The Journal of Physical Chemistry C 115, 5063 (2011).
- Johansson et al. [2005] P. Johansson, H. Xu, and M. Käll, Phys. Rev. B 72, 035427 (2005).
- Dezfouli et al. [2019] M. K. Dezfouli, R. Gordon, and S. Hughes, ACS Photonics 6, 1400 (2019).
- Dunkelberger et al. [2022b] A. D. Dunkelberger, B. S. Simpkins, I. Vurgaftman, and J. C. Owrutsky, Annual Review of Physical Chemistry 73, 429 (2022b).
- Marcus [1964] R. A. Marcus, Annual Review of Physical Chemistry 15, 155 (1964).
- Levich [1966] V. Levich, Adv. Electrochem. Electrochem. Eng 4, 249 (1966).
- Jortner [1976] J. Jortner, The Journal of Chemical Physics 64, 4860 (1976).
- Abendroth et al. [2015] J. M. Abendroth, O. S. Bushuyev, P. S. Weiss, and C. J. Barrett, ACS nano 9, 7746 (2015).
- Ahlberg et al. [1981] E. Ahlberg, O. Hammerich, and V. D. Parker, Journal of the American Chemical Society 103, 844 (1981).
- Olsen and Evans [1981] B. A. Olsen and D. H. Evans, Journal of the American Chemical Society 103, 839 (1981).
- Campos-Gonzalez-Angulo et al. [2019] J. A. Campos-Gonzalez-Angulo, R. F. Ribeiro, and J. Yuen-Zhou, Nature Communications 10, 4685 (2019).
- Colbert and Miller [1992] D. T. Colbert and W. H. Miller, The Journal of Chemical Physics 96, 1982 (1992).
- Hopfield [1969] J. J. Hopfield, Phys. Rev. 182, 945 (1969).
- del Pino et al. [2015] J. del Pino, J. Feist, and F. J. Garcia-Vidal, New Journal of Physics 17, 053040 (2015).
- Xiang et al. [2018] B. Xiang, R. F. Ribeiro, A. D. Dunkelberger, J. Wang, Y. Li, B. S. Simpkins, J. C. Owrutsky, J. Yuen-Zhou, and W. Xiong, Proceedings of the National Academy of Sciences 115, 4845 (2018).
- Xiang et al. [2020] B. Xiang, R. F. Ribeiro, M. Du, L. Chen, Z. Yang, J. Wang, J. Yuen-Zhou, and W. Xiong, Science 368, 665 (2020).
- Morris [2012] C. Morris, The dawn of innovation: The first american industrial revolution (2012) p. 42.
- Richards [2016] V. Richards, Nature Chemistry 8, 1090 (2016).