The Composition and Chemistry
of Titan’s Atmosphere
111Accepted for publication in ACS Earth and Space Chemistry
Abstract
In this article I summarize the current state of knowledge about the composition of Titan’s atmosphere, and our current understanding of the suggested chemistry that leads to that observed composition. I begin with our present knowledge of the atmospheric composition, garnered from a variety of measurements including Cassini-Huygens, the Atacama Large Millimeter/submillimeter Array (ALMA), and other ground and space-based telescopes. This review focuses on the typical vertical profiles of gases at low latitudes, rather than global and temporal variations. The main body of the paper presents a chemical description of how complex molecules are believed to arise from simpler species, considering all known ‘stable’ molecules – those that have been uniquely identified in the neutral atmosphere. The last section of the paper is devoted to the gaps in our present knowledge of Titan’s chemical composition and how further work may fill those gaps.
keywords:
American Chemical Society, LaTeX+1 301 286-6757
NASA GSFC]
Planetary Systems Laboratory, NASA Goddard Space Flight Center
8800 Greenbelt Road, Greenbelt, MD 20771
\abbreviationsIR,NMR,UV
1 Introduction
Titan, Saturn’s largest moon, was first observed in 1655 by the Dutch astronomer Christiaan Huygens. This important discovery that Saturn, in addition to Jupiter, had its own satellite helped to consolidate the Copernican worldview: that the Earth was no longer to be considered the center of the Solar System, but rather one of several planets orbiting the Sun, and possessing a natural satellite of its own. In his excitement at this new finding, Huygens would have little suspected what would transpire 350 years later: that a machine devised and launched into the heavens by humanity and bearing his name would traverse an unimaginable void, and then land softly on his new world, finding it stranger and more alien than even the machine’s designers had anticipated1.

Over the 13 years from 2004 to 2017, the Cassini-Huygens mission2, 3 was able to significantly reveal Titan, both to our eyes and to our minds. Between the successful landing of the ESA-built Huygens probe carrying six scientific suites in January 2005, and the 127 flybys of the NASA-built Cassini spacecraft with its own twelve science instruments, our knowledge of Titan now is vastly greater than before the mission arrived. So, in our privileged position of hindsight, what do we now know about Titan, almost four centuries since its discovery?
First and foremost, it is a moon with a dense atmosphere (Fig. 1), the only such body known in our Solar System. Also that this atmosphere, composed primarily of molecular nitrogen and methane, is a largely anoxic environment, with little oxygen to cause the termination of complex organic reactions. The result is a chemical wonderland, with a breathtaking array of complex organic molecules, of which we presently have only the most rudimentary understanding. Fig. 2 shows a schematic overview of the presumed chemistry that occurs in Titan’s upper atmosphere, where the ‘raw ingredients’ of its photochemical reactions, N2 and CH4, are broken apart and recombined into successively larger molecules, and finally haze particles.

Titan is a world that is today both tantalizingly more known, and more unknown than ever before. Our direct investigations of its atmosphere by the Huygens lander and Cassini spacecraft have both increased our understanding of Titan enormously and also multiplied our questions. Significant outstanding questions include:
-
•
Why is Titan the only moon in the Solar System with a significant atmosphere, and how did it come to be in its present state?
-
•
Is the atmosphere today in a steady state, or is it growing or shrinking or changing in some way?
-
•
To what degree has the atmosphere interacted with and shaped the surface and subsurface?
-
•
What degree of chemical complexity is reached in Titan’s atmosphere, and are precursor biomolecules among the products?
-
•
Are there other moons with atmospheres similar to Titan elsewhere in the galaxy?
Achieving a better understanding of Titan’s atmosphere and its chemistry is important both for the sake of Titan science itself, and because of its potential to inform us about other environments. This includes the present-day Earth, since Titan and Earth are the only objects in the Solar System today to have a hydrological cycle of evaporation, condensation and precipitation, and associated rivers, lakes and seas 4, 5, 6, 7. Like the Earth, Titan also experiences seasons, due to orbiting close to Saturn’s equatorial plane, which is tilted 27∘ to the ecliptic. Titan therefore experiences summer and winter in each hemisphere, seasons that last 7.4 longer than on Earth, with transitional equinox periods of equal daylight at all latitudes.
We also note the relevance to the early Earth, which likely had a much more chemically reducing atmosphere in its distant past 8, 9, 10, 11, 12, 13, before the Great Oxidation Event 14, 15, 16. Finally we can surmise the likely relevance to exoplanets, which vastly outnumber the planets in our own Solar System, and more likely than not include Titan-like bodies somewhere in our galaxy 17, 18, 19.
In this review paper, I attempt to lay out a simple picture of the known characteristics of Titan’s atmosphere, with a focus on the composition and chemistry of the dense lower atmosphere. By necessity, this review will not cover, except in passing, many related areas: the origin of the atmosphere and possible replenishment mechanisms by internal or external sources; isotopic composition and time evolution of isotopic ratios; winds and dynamics; condensates and meteorology; and the chemical composition of large particulates (haze particles). All of these topics have been covered extensively in review articles and chapters elsewhere7, 20, 21, and in two books written about the results of the Cassini-Huygens mission 22, 23.
In presenting a simple overview and summary focusing on the chemistry and composition of the neutral atmosphere, it is hoped that I will do sufficient justice to this one area to make this article a useful primer for undergraduate or graduate students, or others new to the field, to quickly gain a basic understanding of Titan’s bulk atmospheric composition, and why it is that way - at least at the present era.
The paper is organized as follows: I first review basic knowledge about Titan’s atmospheric temperature structure and gas composition. The main section of the paper contains an exposition on the chemistry of the 24 known molecules in the neutral, lower atmosphere. This is followed by a detailed discussion of future research directions in Titan atmospheric composition studies, followed by a Summary and Conclusions.
2 Atmospheric Composition and Structure
2.1 Atmospheric Composition
Titan’s atmosphere is largely composed of two gases: N2 and CH4. The vertical profile of methane comes from measurements by instruments on the Cassini-Huygens space mission, primarily the Huygens GCMS (Gas Chromatograph and Mass Spectrometer) 24 from 0–146 km, the Cassini Visual and Infrared Mapping Spectrometer (VIMS)25 (50–850 km), the Cassini UVIS (Ultraviolet Imaging Spectrometer) 26 (400–1650 km), and the Cassini INMS (Ion and Neutral Mass Spectrometer) 27 (900–1500 km). Their results have been reported in publications from the mission 28, 29, 30, 31.
Aside from noble gases (36Ar, 40Ar and 22Ne) 32, 31, 22 molecular species other than N2 and CH4 have been definitively detected in Titan’s atmosphere at the time of writing (see Fig. 3): ten hydrocarbons (C2H2, C2H4, C2H6, c-C3H2, CH2CCH2, CH3CCH, C3H6, C3H8, C4H2, c-C6H6), eight cyanides222Also referred to as ‘nitriles’ when -CN occurs in an organic molecule. (HCN, HNC, HC3N, C2N2, CH3CN, C2H3CN, C2H5CN, CH3C3N), three oxygen-bearing species (CO, CO2, H2O) plus H2. These gases were originally detected by a variety of astronomical and remote sensing techniques from the ground and space.

All of the major types of hydrocarbons have been detected (alkanes, alkenes, alkynes, a carbene, an aromatic ring). However, major chemical families of nitrogen-bearing molecules (including amines, imines, azines, and N-heterocyclic rings) and oxygen-bearing molecules (such as aldehydes, ketones, alcohols, ethers) are possible ingredients of the atmosphere but remain undetected - a subject we will return to in a later section.
Oxygen has yet to be detected on Titan in an organic molecule such as methanol (CH3OH) or formaldehyde (H2CO), being found so far only in the simple inorganic molecules CO, CO2, and H2O. This limits the presently confirmed scope of astrobiological molecules (i.e. those with the elements CHON in a variety of functional groups) - at least in the atmosphere. At the surface and in the subsurface - where hydrocarbons are thought to be readily hydrolyzed as seen in laboratory experiments 33, 34, 35 - the astrobiological potential may be much greater 36, 37, 38.
The reaction pathways that lead between these molecules have been compiled into computational models of the atmospheric chemistry, which have largely been successful at replicating the observed gas abundances. Models pre-dating the Cassini-Huygens mission 39, 40, 41, 42, 43, 44, 45, 46, 47 primarily focused on replicating the observed neutral gas abundances as measured by Voyager 48 and the Infrared Space Observatory (ISO) 49. However, some models were also developed for the ionosphere 50, 51, 52, 53, 54. During the Cassini-Huygens mission and since, new information collected by the spacecraft, especially from direct sampling of the ionosphere,28, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78 has prompted many new and revised models of Titan’s atmosphere 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103.
At the opposite end of the size scale, molecular growth by covalent bonding and agglomeration results in macro-molecular haze particles 104, 105, 106, 107, 108, 11, 61, 109, 110, 111, 112, composed of thousands to millions of individual atoms 113. As these particles reach a size of 1 m, they begin to sediment (or form the nuclei for condensate growth) and are removed from the atmosphere 114, 115, 116, 117, apparently forming vast dune fields on the surface 118, 119, 120.
2.2 Atmospheric Temperature Structure
Titan’s atmospheric temperature structure (Fig. 4) is a result of the competing heating and cooling processes that take precedence at different altitudes. In the dense lower atmosphere, convection driven by surface heating leads to a vertically decreasing temperature profile, as warm air rises and adiabatically cools. A temperature minimum is reached at 45 km, the tropopause, as confirmed by direct measurements121 from the Huygens Atmospheric Structure Experiment 122 (HASI) and occultations by the Cassini Radio Science Subsystem123 (RSS) 124, 125, 126.

Above the tropopause, heating by absorbed solar energy, primarily by atmospheric haze particles, causes temperatures to rise again in the stratosphere 129. Stratospheric temperatures have been measured by Cassini HASI 121 and by the Cassini Composite Infrared Spectrometer 130 (CIRS) 131, 132, ALMA 133, 134, 128, as well as through radio occultations for the lower stratosphere 124, 125, 126. At around 250–400 km, a temperature maximum, the stratopause, is reached at 180 K 135. The exact altitude (pressure) and temperature of the stratopause varies with both latitude and season 136, 137, 127 over the course of Titan’s long year (29.46 Earth years), being higher and warmer (by 20 K) over the winter pole. This somewhat counter-intuitive result can be understood as resulting from adiabatic compression of air in the descending branch of the global stratospheric Hadley cell.
Temperatures fall throughout the next layer, the mesosphere, as haze becomes thin, and radiative cooling by gases such as HCN and C2H2 becomes increasingly important 129, 135. Titan’s mesopause is reached at 600 km 128, above which altitude temperatures rise again. This is primarily due to methane UV absorption 138, blocking of outgoing IR radiation by C2H6, and far-infrared HCN rotational lines 129. This is the thermosphere, a region where gas collisions are rare, and molecules must wait to spontaneously emit a photon to lose energy.
The temperature structure of the upper atmosphere is highly variable52. Thermal oscillations of significant amplitude were inferred by Huygens HASI121 above 500 km, while \latinin situ measurements of electron temperature (by the Cassini Radio and Plasma Wave Spectrometer - RPWS 139) and density and composition (by INMS) have shown significant time variability on diurnal 66, 140, 141 and longer timescales depending on the level of solar activity 142 as well as the position of Titan within Saturn’s magnetosphere 143, 144, 145, 146. More recently ALMA measurements are now able to probe the thermal structure of the upper atmosphere as well, providing the ability to monitor secular changes over time.128, 147
Above the four layers of the bound atmosphere is the exosphere, beginning at the exobase (1500 km 148), a region where gases can freely escape to space. These five regions mirror the temperature structure of the Earth’s atmosphere, but with a substantially larger scale height (approximately 5) due to the lower surface gravity. Overlapping the upper thermosphere is the ionosphere ( km), defined as the region where "significant numbers of free thermal (1 eV) electrons and ions are present." 149
It is important to note that the vertical profiles of temperature, minor gas abundances and haze density all vary with both latitude and season. Titan - like the Earth and other planets with atmospheres - exhibits one or more convection cells in the middle atmosphere. Near to the equinoxes, air rises at mid-latitudes and flows to both poles, where it descends and then returns equator-ward 150, 151, 152, 153, 154. However, close to the solstices the circulation more closely resembles a single cell with flow from the summer to winter hemisphere. These cells act to redistribute thermal energy, trace gases and hazes in both altitude and latitude. This topic has been the subject of extensive measurements and modeling in the literature (e.g. 127, 155, 156, 157, 158, 159, 160, 161, and references therein). In this review I will not further discuss latitudinal or longitudinal variations in atmospheric structure and focus only on the vertical chemistry variations typical of mean conditions at low latitudes.
2.3 Gas Vertical Profiles
Fig. 5 shows typical vertical profiles of the 24 known molecular species at low latitudes, compiled from a combination of ground and space-based measurements, and some photochemical model profiles constrained by observations. These include two pairs of structural isomers: HCN and HNC, CH3CCH and CH2CCH2.

Some important trends can be noted. Methane is an unusual outlier, with a greater abundance in the troposphere ( km) than above. This is due to the ‘cold trap’ effect, where it reaches saturation as the tropospheric temperature drops with altitude, and therefore its mixing ratio is reduced as it forms clouds at 15-30 km. The gases N2 and CO are well-mixed, having approximately uniform profiles throughout the atmosphere due to long photochemical lifetimes. Two other gases: H2 and C2H4 also do not condense at the ‘cold trap’ - the coldest part of the atmosphere around the tropopause at 45 km (70 K). The profile of H2 is shown here as constant at the 0.1% value typical of the lower stratosphere, since measurements of its vertical profile remains uncertain.
All other gas species show profiles that typically decrease downwards from the upper atmosphere, due to having a source due to photochemistry at high altitudes, and then becoming diluted as they are mixed downwards into the denser part of the atmosphere. In many cases the actual measured profiles are still rudimentary, constrained by only a few data points and with even less knowledge of meridional and temporal variations. Some gases may exhibit increases again towards the stratosphere, due to either secondary production peaks (e.g. due to cosmic ray deposition) or due to redistribution by atmospheric circulation.
3 Atmospheric Photochemical Processes
In this section I present a brief overview of the types of reactions that occur in Titan’s atmosphere, as a prelude to the discussion of the chemistry of individual molecules in the following section (see also Table 5 of Vuitton et al. 103 and description therein). Different chemical processes become important at different levels of the atmosphere, due to the altitude variation of temperature, density and penetration depths of charged particles and photons that affect the reactions. For example, Saturn magnetospheric electrons are stopped high up in the ionosphere, while solar photons penetrate to varying depths depending on wavelength.103 High energy cosmic rays are the deepest-penetrating rays in the atmosphere, peaking in energy deposition at around 100-150 km altitude.103 The various processes, depicted in Fig. 6, are now examined in more detail.

3.1 Photodissociation and electron impact dissociation
Dissociation (break-up, or fragmentation) of a molecule occurs when a sudden influx of energy breaks molecular bonds. In Titan’s atmosphere this happens readily in the upper atmosphere (700 km), due to both energetic UV solar photons (h) and by the impact of fast-moving electrons () trapped in Saturn’s magnetic field 172, 173 (Fig. 2).
Dissociation often leads to neutral (uncharged) molecular fragments, which may either be in the ground state or excited states, e.g.:174, 175, 176
| (1) | |||||
| (2) |
where is ground state methylene and is an excited state. Note that other possible fragmentation products are possible, the examples given above are only one possibility in each case. Also note that for simplicity, electronic states of molecules are usually omitted in this paper, unless required to distinguish between two otherwise identical reagents that have significantly different properties.
The molecular fragments are often radicals – i.e. highly reactive atomic or molecular species that have unpaired electrons, such as H, CH, and N. These radicals are quick to react with other radicals, or with neutral species.
3.2 Ionization
Instead of breaking up (dissociation), a molecule may instead become ionized (positively or negatively charged), typically by losing an electron to become a cation, e.g.: 181, 182, 29
| (3) | |||||
| (4) |
| (5) | |||||
| (6) |
| (7) | |||||
| (8) |
| (9) |
3.3 Ion reactions
Dissociative recombination is the process whereby a positive ion reunites with an electron, and in the process breaks apart. An example in Titan’s atmosphere is: 188, 189
| (10) |
Radiative association is a reaction whereby two species combine, shedding excess energy via a photon. These reactions typically occur only in rarefied environments ( mbar190 where a metastable intermediary complex has time to form and then stabilize by emission of a photon. Despite being impossible to observe in laboratory conditions due to the long lifetimes of the intermediate states191, 192, such reactions are thought to occur in interstellar clouds193, 194, 195, 196, as well as in Titan’s upper atmosphere. Radiative ion-neutral association reactions on Titan may include: 197, 103
| (11) | |||||
| (12) |
| (13) | |||||
| (14) |
Ions may also react with each other, leading to neutral products, although Vuitton \latinet al.103 argued that positive-negative ion recombination rates are too small to compete with ion-neutral reaction pathways.
3.4 Radical reactions
Radicals (molecules with an unpaired electron) react with other radical and non-radical species in multiple ways. Radicals may react with each other in association reactions 47:
| (15) |
Radicals may attack neutral molecules, for example in the case of hydrogen abstraction, which is a major loss process for methane 41, 199, 200, 103:
| (16) |
Other examples include substitutions of terminal atoms or groups, typically at carbon-carbon double or triple bonds 201, 202, 203:
| (17) | |||||
| (18) |
| (19) |
Three-body association reactions occur when two reactants meet to form a metastable, intermediate complex that is then stabilized by collision with a third, non-reacting body that carries away energy, allowing the metastable complex to stabilize. The two steps are:
| (20) | |||||
| (21) |
where the asterisk is used to denote an excited state. In this paper I typically simplify such reactions to a single step:
| (22) |
Such reactions are of critical importance to the formation of many hydrocarbons, especially alkanes, e.g.207, 208, 197:
| (23) | |||||
| (24) |
Note that three-body reactions are limited to Titan’s dense, lower atmosphere where there is a sufficiently high collision rate to allow the collisional stabilization to occur.94
Radiative association reactions may also occur between radicals. Vuitton et al.94 studied the effect of radiative associations on Titan’s chemistry and proposed that reactions such as:
| (25) | |||||
| (26) |
may occur in Titan’s atmosphere.
3.5 Other reactions
Molecules may also re-organize their structure to become more stable, such as in collisional isomerization. Two important known instances of this on Titan are the conversion between propadiene (allene) and propyne by H atoms,41, 99 with a barrier of 65.1 kcal/mol:209
| (27) |
| (28) |
Polymerization is the process where multiple similar, unsaturated hydrocarbons join together form linear chains:
| (29) |
4 Chemistry of the Neutral Atmosphere
In this review, I focus on the 24 molecules detected in Titan’s dense, neutral atmosphere (Fig. 7). Many other neutral species have been inferred from ion and neutral mass spectroscopy in Titan’s upper atmosphere 28, 58, 219, 61, 220, 65, through photochemical models 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103 and via laboratory experiments (see review by Cable \latinet al.111). The choice to limit the discussion to the chemistry and composition of the 24 definitively identified molecules of the neutral atmosphere was made for several reasons: (i) this set of molecules includes the most easily detectable and likely the most abundant molecules of the atmosphere, which therefore provide a good overview of bulk chemistry and composition; (ii) other molecules inferred only through single-stage mass spectroscopy do not have robust structural identifications, since this technique cannot typically distinguish between isomers having the same chemical formula, occurring at the level of complexity of three carbon atoms and beyond; (iii) to allow for a more detailed discussion of the molecules that have been unambiguously detected; and (iv) because the composition of the neutral, lower atmosphere is the most important chemical inventory for consideration of other processes such as condensation and meteorology, sedimentation to the surface, and astrobiology at Titan’s surface and interior.

Note on chemical names: the topic of chemical nomenclature remains eternally problematic. For example, the relatively simple compound C2H3CN has been commonly referred to as ‘vinyl cyanide’ in most 20th century astronomical literature, though preference has shifted more recently towards the simpler, single-word name ‘acrylonitrile’ (also with ‘methyl cyanide’ to ‘acetonitrile’). In fact multiple valid names for C2H3CN exist, including ‘2-propenenitrile’, ‘cyanoethene/ cyanoethylene’ and ‘propenenitrile’, however it must be noted that the preferred official (IUPAC) name is actually the rather cumbersome ‘prop-2-enenitrile’.
IUPAC molecular nomenclature certainly has its place. However, for the purposes of discourse in planetary atmospheric chemistry, dominated by small molecules, the formulaic names for molecules can be not only inconvenient, but an actual obstacle to reading and digesting information. Therefore, in this work I have followed a naming convention based on a combination of modernity, modified in places for greater simplicity, i.e.: (a) single word names are generally preferred over multiple word names (e.g. ‘propyne’ over the older standard ‘methyl acetylene’); (b) avoiding numbers in names of small molecules except where necessary, and (c) accepting that some names are close enough to be interchangeable (e.g. there is little confusion engendered by using either ‘ethylene’ and ‘ethene’; ‘propylene’ and ‘propene’ etc).
While I run the risk of offending practicing chemists, I hope that the terminology is consistent enough for readers to understand what molecule is being referred to, and convenient enough for simplified writing in typical planetary science usage.
4.1 Hydrocarbons and Hydrogen
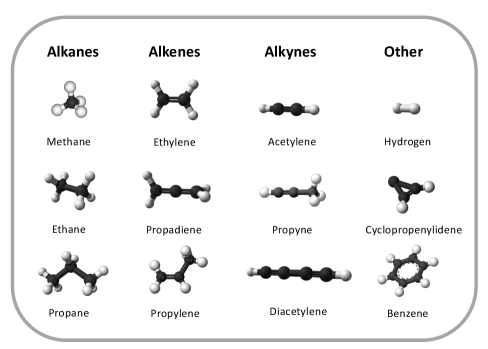
Hydrocarbons are molecules formed from atoms of only hydrogen and carbon. Due to the four-fold valency of carbon, many bonding configurations are possible. The most common families of aliphatic (acyclic) hydrocarbons include (i) the alkanes - where carbon is saturated having four single-bonds; and the two unsaturated types - (ii) alkenes, featuring carbon-carbon double bonds, and (iii) alkynes with carbon-carbon triple bonds. Once there are four or more carbon atoms, mixed types become possible (see Fig. 8).
Cyclic hydrocarbons occur where there are one or more closed rings of carbon atoms (at least three are needed to make a ring, or cycle). Cyclic molecules that have a pi bond of delocalized electrons are known as ‘aromatic’ (small aromatics are typically volatile at room temperature), although not all rings are aromatic. For example the unsaturated six-carbon ring benzene (c-C6H6) is a well-known aromatic, whereas its saturated cousin cyclohexane (c-C6H12) is a non-aromatic cycle. Carbenes are reactive molecules where carbon has two unbonded but paired electrons, such as methylene (CH2).
Hydrocarbon molecules are created through the breakup and recombination of fragments of methane. A prime example is the formation of ethane from two methyl radicals:221, 222
| (30) | |||||
| (31) |
Note that, in the process, significant amounts of molecular hydrogen will form from the photo-dissociated hydrogen atoms 223:
| (32) |
leading to the trace amounts of hydrogen (0.1%) found in Titan’s lower atmosphere. Note that this termolecular reaction is an important process leading to the creation of H2 in the Interstellar Medium (ISM).
Significant loss of hydrogen to space is thought to occur,40, 224, 148, 225, 226, 227, 228 preventing methane from being recycled, as occurs on the giant planets, leading to gradual depletion of methane in Titan’s atmosphere in the absence of outgassing or other replenishment.41, 229, 230, 231, 232

A network diagram showing the principal neutral pathways for hydrocarbon molecule formation is shown in Fig. 9. Hereafter follows a high-level description of the key chemistry for each of the hydrocarbons and hydrogen leading to their relative abundances in the neutral atmosphere.
4.1.1 Hydrogen

Detection: Molecular hydrogen (H2) was was tentatively detected by Trafton233 using the 107 inch telescope at the McDonald Observatory, via absorption in the S(0) and S(1) quadrupole lines of the 3-0 band at 0.82 m. Hydrogen was clearly detected in the far-infrared by the Voyager 1 IRIS spectrometer 234 and confirmed by Cassini CIRS 235. The H2 Volume Mixing Ratio (VMR) was measured directly by the Cassini INMS instrument in the ionosphere as 0.4% 28 and in the lower stratosphere and troposphere by Huygens GCMS at 0.1% 31. The fact that hydrogen, presumed to be produced in the upper atmosphere by photolysis of methane 40, 41, was measured to have a decreasing abundance downwards, has proved difficult to replicate in models. Models have required a sink for H2 at the surface 225, 135, 236, which has even been suggested as possibly biological in origin 237. Important reactions for hydrogen are shown in Fig. 10 238.
Production: Molecular hydrogen can be produced in ion-phase reactions such as239:
| (33) | |||||
| (34) | |||||
| (35) |
| (36) | |||||
| (37) |
Loss: H2 may be lost to photolysis, yielding 2H, although Vuitton \latinet al.103 has argued that this is a relatively small source of H in Titan’s atmosphere, due to shielding of H2 by CH4 and N2, with most H-production coming from photolysis of methane.
| (38) | |||||
| (39) |
| (40) | |||||
| (41) |
4.1.2 Methane

Profile: Methane (CH4) was the first molecule to be positively identified in Titan’s atmosphere, via visible and near-IR absorptions seen by Gerard Kuiper 246, using the 82 inch reflector at McDonald Observatory. We now know that methane is the basic ingredient enabling all of Titan’s complex organic (i.e. carbon) chemistry, allowing reactions to proceed up to the creation of haze particles: some key reactions for methane are shown in Fig. 11247, 248.
Nevertheless, methane may be a gradually depleting resource since it is not permanently recycled, unless replenished by an as yet unidentified mechanism 41, 249, 232, 231, 250. Speculative mechanisms include: crustal destabilization leading to outgassing from methane clathrates,251 outgassing from cryovolcanism,252, 253, 254 and displacement from near-surface clathrate materials by condensed ethane255 - yet observational evidence for these processes remains inconclusive at best.
Methane’s vertical profile can be divided into three zones: (i) a tropospheric zone where the fractional abundance gradually decreases from 5.5% at the surface to a minimum at the tropopause of around 1.4%, due to reaching saturation at decreasing volume mixing ratios (VMRs) as the temperature decreases towards the tropopause (‘cold trap’); (ii) a relatively constant amount of 1.4% in the stratosphere, mesosphere and thermosphere; (iii) a gradually increasing mixing fraction above an altitude of 800–850 km (the methane homopause) in the increasingly collisionless regime, due to the differing scale heights of different molecules 135. It is presently uncertain whether methane has any variation with latitude on Titan, although a variation from 1.0% to 1.5% in the lower stratosphere has been reported 256 based on infrared measurements by Cassini CIRS.256
Loss: The photolysis of methane in the upper atmosphere leads to the formation of radicals including methyl (CH3), methylidene (CH), and the carbene methylene ( or )103 which undergo a chain of reactions to form all the hydrocarbons found in Titan’s atmosphere. Note that as much as 75% of methane photolysis above 700 km is due to the solar Lyman- line at 121.6 nm.257
The fate of most methane is ultimately to form ethane via the addition of two methyl radicals (R 31) or via the creation of ethyl:
| (42) |
leading to permanent loss of methane. Methyl radicals are produced either directly by primary photolysis, or by reaction of methane with radicals:205, 258
| (43) | |||||
| (44) | |||||
| (45) |
Note that the reaction of the ethynyl and vinyl radicals (amongst others) with methane is a catalytic destruction process, since the acetylene and ethylene generated are easily photolyzed back to the radical form where they can continue to destroy methane molecules. This process may be repeated hundreds of times before the radical catalysts are themselves lost to form higher hydrocarbons. This is the main source of methane depletion in Titan’s atmosphere.103
Methane fragments participate in ion chemistry, first being ionized by charge transfer:242
| (46) | |||||
| (47) |
and then building up ions, e.g.:239
| (48) |
Future directions: Although the chemistry of methane is perhaps one of the best-understood for any molecule on Titan, the most pressing questions remain the nature of its origin, and possible replenishment.21
4.1.3 Acetylene

Acetylene was the third molecule to be identified in Titan’s atmosphere,259 following the detection of methane and ethane, when Gillett observed its 13 m band in mid-infrared spectroscopy with 2 m and 4 m telescopes at Kitt Peak Observatory. Important reactions for acetylene are shown in Fig. 12.260, 261, 257, 103
Production: Acetylene may be produced either directly or indirectly from photolysis products of methane. In a direct process262:
| (49) |
| (50) | |||||
| (51) |
| (52) | |||||
| (53) |
Loss: In the ionosphere, photolysis of acetylene, and electron transfer to N produces 242, which reacts with neutrals to build heavier ions239, e.g.:
| (54) | |||||
| (55) |
which may (dissociatively) recombine with to form neutral species266, 267. Likewise, proton transfer to neutral acetylene leads to , which can also form species239:
| (56) | |||||
| (57) |
Finally, neutral C2H2 in the ionosphere may combine with other ions to build heavier species239, e.g.:
| (58) | |||||
| (59) |
In the neutral atmosphere, acetylene is lost by reaction with methylene forming propargyl268, 269, 270:
| (60) |
Acetylene absorbs photons to longer wavelengths (230 nm79) than methane, so its photolysis continues into the stratosphere (see Fig. 12). This produces ethynyl (C2H) and carbyne (C2) which are potent means of methane depletion via hydrogen abstraction:223
| (61) | |||||
| (62) |
The acetylene produced by this reaction is recycled back to ethynyl by photolysis, and thereby each acetylene/ethynyl may cause the loss of hundreds of methane molecules before being lost itself to another reaction pathway such as:271
| (63) |
Catalytic destruction of methane in this way is the principle means of methane depletion in Titan’s atmosphere 41, 79, 103. In the lower atmosphere ( km), the dominant loss process for acetylene is conversion to ethylene by a two-step reaction with atomic hydrogen:94, 103
| (64) | |||||
| (65) |
Future directions: Acetylene has been detected on Titan’s surface 32, 272, and is likely to be present in the northern lakes and seas 273, 274. Future investigation by the mass spectrometer (DrAMS) instrument of Dragonfly275 will further refine the surface and near-surface abundance.
C2H2 was one of the first molecules investigated to form a co-crystal 276, 277 at Titan surface temperatures, an organized co-condensate of two or more chemical species. The validity of multiple co-crystal types has since been established, but requires further laboratory work to determine the full parameter space of possible crystalline types.
4.1.4 Ethylene

Ethylene (C2H4) was discovered by infrared spectroscopy at the same time as acetylene 259. The vertical profile of ethylene exhibited a surprising trend to decrease in abundance upwards in the lower stratosphere early in the Cassini mission163, 278, although this faded at later seasons 137, 127.
Ethylene is a crucial, two-carbon neutral molecule that provides a stepping stone from methane to higher hydrocarbons (Fig. 13)279, 280. Ethylene is remarkable in being one of the few molecules (along with H2 and N2) that does not condense at the tropopause, and therefore persists in significant quantities into the troposphere.
Production: Ethylene may be produced in the ionosphere by dissociative recombination of heavier ions with an electron281, 282, 266:
| (66) | |||||
| (67) |
In the neutral atmosphere, ethylene is largely formed through reactions between methane and its derived radicals, or between radicals283, 257, 262, 79, 268, 269:
| (68) | |||||
| (69) | |||||
| (70) |
At lower altitudes, production through the intermediary is also important223:
| (71) | |||||
| (72) |
Loss: In the ionosphere, the ethylenium ion - produced from ethylene photoionization - can be lost in various reactions with neutrals239:
| (73) | |||||
| (74) | |||||
| (75) |
and ethylene can be lost during the formation of heavier ions239:
| (76) | |||||
| (77) |
Photolysis of ethylene leads to acetylene (R50 and R51). Insertion/addition reactions onto ethylene by CH, for example, can lead to higher hydrocarbons283, 204, 284:
| (78) | |||||
| (79) |
Below 500 km, loss via H-addition becomes important94:
| (80) |
The CN radical can also substitute onto ethylene to create vinyl cyanide (also known as acrylonitrile, or propenitrile):98, 202, 203
| (81) |
Future directions: Ethylene is the simplest alkene, the family of hydrocarbons having a double CC bond. Photolysis or other breaking of the alkene CC bond leads to radicals which rapidly react, leading to formation of polymers. The role of polymer formation in Titan’s atmosphere is incompletely understood, but is likely to be an important process in the formation of haze particles.
4.1.5 Ethane

Ethane was the second molecule to be discovered in Titan’s atmosphere, via its strong band at 12 285 seen by Gillett with the 60-inch telescope on Mount Lemmon. Ethane forms one of the primary trace gases in Titan’s atmosphere with concentrations greater than 1 ppm in the stratosphere 48, 286, 287, and is the primary sink for methane loss 41, 47. Due to this observation, a global deep ethane ocean was originally predicted 288, 289 but later proved not to be the case 290, 291.
The liquid hydrocarbon bodies eventually detected on Titan’s surface292 have a measured ethane content that varies between different seas and is generally less than the methane fraction 293, 294, except for the southern lake Ontario Lacus 295, 296. Ethane may form co-crystals in Titan lakes with other organics, such as benzene 297. Ethane has also been implicated in displacing methane from clathrate hydrate, allowing for a partial resupply mechanism of methane to the atmosphere 255, which is otherwise continuously lost by chemistry 41, 298.
Production: Ethane is primarily produced by the addition of two methyl reactions (R31) but also reforms from the ethyl radical299:
| (82) | |||||
| (83) |
Loss: Ethane can be lost through photolysis back to , or to stable molecules such as ethylene and acetylene with loss of hydrogen (see Fig. 14). Ethane can also be attacked by reactive radicals such as methylene, methylidene and ethynyl (from acetylene photolysis)223, 79, 283, 204:
| (84) | |||||
| (85) | |||||
| (86) |
Ethane may form heavier ions through reactions such as239:
| (87) | |||||
| (88) | |||||
| (89) | |||||
| (90) | |||||
| (91) |
Future directions: Large amounts of ethane are thought to condense in Titan’s lower stratosphere, and form a significant fraction of Titan’s lakes and seas 273, 303. Ethane was implicated in the formation of a vast north polar cloud seen during northern winter in 2005 by Cassini VIMS 304, although other interpretations have suggested that this cloud is condensed methane.305 While the basic chemistry of ethane is well-understood, an improved understanding of its condensation - especially co-condensation with other gases - will be crucial to a more accurate interpretation of Titan’s meteorology.
4.1.6 Cyclopropenylidene

Cyclopropenylidene (c-C3H2) is the first carbene (a molecule having two unbonded, self-paired valence electrons from a carbon atom) and the second cyclic molecule to be found in Titan’s atmosphere (after benzene). c-C3H2 was detected using millimeter wavelength astronomy with ALMA 165, the third molecule whose first detection on Titan was achieved with with this telescope.
Production: In the upper atmosphere, production of a precursor, the cyclopropenyl cation () is thought to proceed by103:
| (92) | |||||
| (93) |
which then recombines with to produce c-C3H2 306 and H.
Other possible pathways include photolysis of propargyl:
| (94) |
In the neutral atmosphere the dominant production pathways may include CH addition to acetyene 307, 308 (see Fig. 15)309, 310:
| (95) |
and below 600 km:311
| (96) |
c-C3H2 can also result from collisional isomerization from its isomers propynylidene (t-C3H2) and propadienylidene (l-C3H2):311
| (97) | |||||
| (98) |
| (99) | |||||
| (100) |
which in turn may dissociatively recombine with an electron, returning to c-C3H2, or splinter into smaller acyclic fragments:266, 313
| (101) | |||||
| (102) | |||||
| (103) | |||||
| (104) |
In the neutral atmosphere above 600 km, c-C3H2 photodissociates to form c-C3H, l-C3H, and C3. It is also lost by reaction with CH3 to form acylic species:311
| (105) |
Below 600 km, these pathways continue to be significant, but there is additional c-C3H2 loss via:311
| (106) | |||||
| (107) |
Future work: Chemical pathways leading to and from c-C3H2 in Titan’s atmosphere remain to be explored, especially given the multiple possible structures for , including propynylidene ( ), propadienylidene (), cyclopropyne () and propenediylidene () 309, 310. Experimental and theoretical work on reaction pathways, rates and branching rates during photolysis will greatly help to clarify the production, loss and stability of cyclopropenylidene.
Several isomers of have been detected in space, including H2CCC 314, which should prompt further astronomical observations to determine if these isomers also exist in Titan’s upper atmosphere. Intriguingly, the CHCCH form has been shown to dimerize to form para-benzyne 307, discussed in a later section.
4.1.7 Propyne

The propyne (CH3CCH, methyl acetylene) isomer of () was first detected in the infrared by the Voyager Infrared Interferometer Spectrometer (IRIS)315 via long wavelength infrared emission bands at 328 and 633 cm-1316.
Production: Propyne and its symmetric isomer propadiene (CH2CCH2, allene) are produced by addition of CH into ethylene (Eq. 79, Fig. 16)317, 318, 319, but also through H-addition to propargyl320:
| (108) |
| (109) |
In the ionosphere, the C3H ion is a precursor to C3H4, and produced via:219
| (110) | |||||
| (111) |
| (112) | |||||
| (113) |
Loss: In the ionosphere, propyne is lost through ion reactions such as239:
| (114) | |||||
| (115) | |||||
| (116) |
Propyne may also be lost by photolysis in the upper atmosphere324:
| (117) |
and to three-body reactions103:
| (118) |
Future work: Many reactions forming or depleting have uncertain branching ratios between CH3CCH and its isomer CH2CCH2. Further work is needed to improve knowledge of these quantities. Collisional interconversion between the two isomers may be mediated by atomic hydrogen99, so accurate measurement of both isomers may be a way to provide constraint on the abundance of otherwise short-lived and difficult to measure H atom.
4.1.8 Propadiene

Propadiene is a less abundant and less thermodynamically stable isomer of C3H4, which is more abundant in Titan’s atmosphere in the form of propyne. Propadiene (CH2CCH2) was detected in Titan’s atmosphere using high-resolution ground-based spectroscopy at NASA’s Infrared Telescope Facility (IRTF) with the Texas Echelon Cross Echelle Spectrograph (TEXES) instrument via its band at 845 cm-1 325.
Production: Like propyne (CH3CCH), propadiene is produced in the upper atmosphere by CH addition to ethylene (Eq. 79, Fig. 17)326, 318, 327, 328, and by H addition to C3H3:320
| (119) |
Lower in the atmosphere, where propene is more plentiful, it can be photodissociated to produce propadiene (see Fig. 18)329:
| (120) |
Ion formation pathways of CH2CCH2 are less certain, but may follow similar channels as propyne, with branching ratios that are currently uncertain.
Loss: Propadiene is lost to direct photolysis318:
| (121) |
to ion reactions such as103:
| (122) |
| (123) |
Future directions: As with propyne, the branching ratios of reactions implicated in the formation of isomers remain uncertain, so further experimental and theoretical work is required. Accurate measurement of both propyne and propadiene is a possible means to indirectly inferring the abundance of atomic hydrogen99 in the lower atmosphere, in the absence of direct, in situ measurements.
4.1.9 Propene

Propene (C3H6, propylene) was first detected using data from the Cassini CIRS infrared spectrometer via its band emission near 11 m 330, 171.
Production: Ion reactions lead to creation of propene from , :239
| (124) | |||||
| (125) |
Propene is predicted to be produced in the upper atmosphere by both H addition to (62%), and CH insertion into ethane (38%)91:
| (126) | |||||
| (127) |
and also lower in the atmosphere by the termolecular reaction103:
| (128) |
Loss: Propene is lost both through photodissociation (Fig. 18331, 332, 333, 334, 321, 329) and ion reactions e.g.:239
| (129) | |||||
| (130) |
In the lower atmosphere, propene is predicted to be lost through a termolecular reaction with H atom addition:103
| (131) |
Future directions: Propene, as an alkene, may also undergo polymerization to form polypropylene, a notable and widespread plastic used on Earth. Most likely polyynes in Titan’s atmosphere are not pure polymers of a single repeated monomer type (ethylene, propylene, etc) but an assorted mixture of many types, with the lighter, more abundant alkenes more heavily represented than larger, heavier units. Further research into polymerization of mixed monomers will yield insights into the formation of Titan’s haze.
4.1.10 Propane
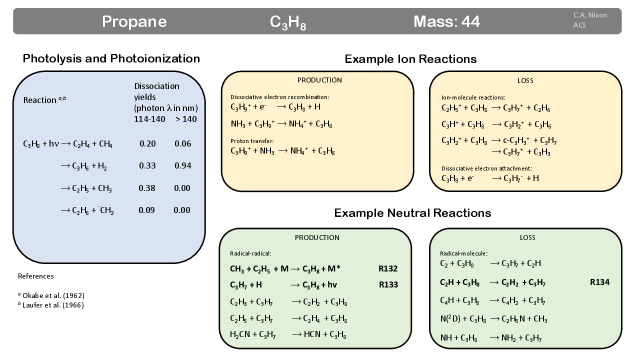
Propane (C3H8) was detected contemporaneously with propyne (CH3CCH) by Voyager’s IRIS instrument 316 via an infrared band at 748 cm-1 and subsequently confirmed by ground-based observations335 and with Cassini CIRS 286, 163, 336.
Production: A significant pathway for the production of propane is by addition of to (Fig. 19)337, 338:
| (132) |
| (133) |
Loss: Propane is primarily lost in the upper atmosphere by photolysis to propene, but participates in other reactions as shown in Fig. 19. Propane also undergoes H-abstraction by ethynyl to recycle acetylene:340
| (134) |
but the fate of is largely to react with H to reform propane.103
Future directions: While the chemistry of propane remains relatively well known, its role in cloud formation and lake composition on Titan remains to be fully explored. Quantum mechanical analysis of propane’s 23 infrared active bands336 remains incomplete, preventing accurate modeling at high resolution for these bands. However in recent years the pseudo-linelist technique has proved useful for providing practical absorption coefficients across a wide bandwidth for calculation at medium resolution 341.
4.1.11 Diacetylene

The presence of diacetylene (C4H2, butadiyne) was inferred from infrared spectroscopy of Titan’s atmosphere with Voyager’s IRIS instrument 342 via emission bands at 220 and 628 cm-1. At present, it remains the only C4 hydrocarbon species confirmed in Titan’s atmosphere (although note that the nitrile CH3C3N, detected with ALMA168, also has four carbon atoms.)
Production: Diacetylene can be produced by the aforementioned reaction of the ethynyl radical with acetylene (R 63), or by stepwise addition to acetylene 283, 343, 103:
| (135) | |||||
| (136) | |||||
| (137) |
Loss: Diacetylene may undergo ionization to (Fig. 20)344, 345, and subsequent loss to processes such as:239
| (138) | |||||
| (139) |
| (140) | |||||
| (141) |
Insertion of ethynyl is a way to lengthen the polyyne chain from diacetylene to triacetylene:347
| (142) |
Future directions: To date, the triacetylene molecule () has remained elusive on Titan, despite its detection in space at high relative abundances compared to C4H2 348. Detection of triacetylene would help to clarify the importance of the radical, which contributes to the depletion of methane in photochemical models along with the smaller related radicals and , as well as the efficacy of polyyne formation in general.
4.1.12 Benzene
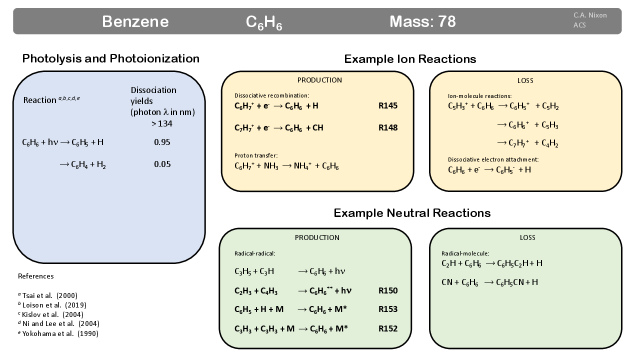
Benzene (c-C6H6, Fig. 21)349, 102, 350, 351, 352 was the second new species detection on Titan made by the Infrared Space Observatory (ISO) in 2003 49, via its strong hydrogen bending mode at 674 cm-1. Benzene was the first cyclic (closed ring) molecule to be detected on Titan, and remains the only confirmed aromatic molecule (molecules with a de-localized electron orbital). The detection of benzene is highly significant, since it provides a measurement of the basic six-membered ring from which larger, multi-ring molecules can be formed,353, 354 building towards macromolecular haze particles - see discussion later in this article.
Production: In the upper atmosphere (800-950 km), a significant pathway to creation of benzene is dissociative recombination (DR) of the phenylium ion. Phenylium is created through:92, 102
| (143) | |||||
| (144) |
which then forms benzene through:355
| (145) |
A second source roughly equal in importance is thought to be dissociative recombination of the C7H ion (benzylium or tropylium, Fig. 22):102

| (146) | |||||
| (147) |
followed by:
| (148) |
Yet a third ion channel is the DR of C8H with leading to benzene plus other hydrocarbon fragments.102
Radical chemistry also leads to to benzene, such as C2 addition to 1,3 butadiene proposed to occur in the ISM:356
| (149) |
An alternate pathway:
| (150) |
also leads to benzene, but in a highly excited state where it will mostly dissociate to C6H5 + H.102 Recently, a new pathway via a smaller, five-membered ring radical (cyclopentadienyl Fig. 22) molecule has been proposed by Kaiser et al.354:
| (151) |
but this has yet to be added to photochemical models to assess its relative importance.
At higher pressures lower in the atmosphere, the three-body reaction combining two propargyl radicals becomes the dominant pathway for creation of benzene: 79, 80, 92, 102
| (152) |
Loss: Benzene is lost through ionization to the phenylium ion (C6H) and through photolysis to form phenyl ()92, 357. The phenyl radical then either reforms benzene:94
| (153) |
or reacts with other radicals and neutral species, leading to molecules such as toluene (), styrene () and benzonitrile ().358
4.2 Nitrogen Compounds
Nitrogen compounds are formed by chemical combination of dissociation products from initial N2 and CH4, and have formulas . All of the eight known heteroatomic nitrogen compounds are cyanides, wherein nitrogen is bonded to carbon by a triple bond (-CN) and therefore have a formula: -. These are HCN, HNC, CH3CN, C2H3CN, C2H5CN, HC3N, CH3C3N and C2N2. Other than the light molecules HCN and HNC, the remaining molecules are nitriles (organic cyanides). See Fig. 23.

Hydrogen isocyanide (HNC) is less stable than hydrogen cyanide (HCN) and is converted exothermically to HCN as it descends in the atmosphere. This leads to a predicted steep decrease in abundance with increasing pressure 359 and its present non-detection at lower altitudes.
In the lower atmosphere, nitrogen has always been found to date to be triple-bonded in the terminal position of a molecule: other types of species (amines, imines etc) have not yet been detected. We will return to the topic of what additional nitrogen compounds may be waiting to be discovered in a later section. The chemistry of known N-bearing molecules in the neutral atmosphere is now summarized.
4.2.1 Nitrogen

A major but unobserved constituent in Titan’s atmosphere was necessitated by the observed collisional broadening of methane spectral lines 360: this was hypothesized to be molecular nitrogen 361 which would be invisible at visible and longer wavelengths. The first conclusive observations of nitrogen were by Voyager 1’s UVS instrument, which detected dayside airglow at 96 and 98 nm, and longer wavelength absorptions with occultation measurements 362, 363. Measurements of nitrogen were greatly extended by Cassini’s UVIS instrument 26, 364, 365, 366, 367.
Production: The origin of nitrogen in Titan’s atmosphere has been long debated, and is not the subject of this paper. In brief, two major theories exist: enclathratization of N2 gas in the protosolar nebula368, or accretion in the form of NH3 ice followed by later photodissociation to eventually form N2 through a reaction cascade 369:
| (154) | |||||
| (155) | |||||
| (156) | |||||
| (157) |
The latter scenario is currently favored due to the low temperatures in the sub-nebula required to capture molecular nitrogen directly. Variations on the theory include impact conversion of either NH3 or ammonium sulfate () to N2 370, 371.
| (158) | |||||
| (159) |
| (160) | |||||
| (161) |
Loss: Molecular nitrogen is dissociated and/or ionized by short-wavelength solar radiation at nm 374, Saturn magnetosphere electrons 375 and Galactic Cosmic Rays (GCRs)177, 178, 179, 180 (Fig. 24)173.
| (162) | |||||
| (163) | |||||
| (164) |
and can recycle to through reaction with hydrogen, methane and other hydrocarbons, e.g. 242:
| (165) | |||||
| (166) | |||||
| (167) | |||||
| (168) |
However, molecular nitrogen in the un-ionized state has very low reactivity, which in part contributes to its great abundance and significant longevity in the atmosphere.
Isotopes: Since and have significantly different UV cross-sections 377, it is important to correctly account for both isotopes and the wavelength variation of the solar spectrum to arrive at correct dissociation rates. Self-shielding by the more abundant is thought to reduce photolysis rates relative to the less abundant, less shielded , causing a lower ratio in nitrogen atoms than in the original molecules. Since significant amounts of atomic nitrogen go on to form nitriles, this skew towards increased production of may explain the lower in nitriles than in N2 itself 377, 378, 379.
Future directions: The dissociation and reaction pathways for N2 and its daughter ions and radicals remain one of the better known areas of Titan chemistry. However gaps remain, in particular whether nitrogen exists in chemicals such as amines and imines, or if it is incorporated in to heterocyclic ring molecules. This is further discussed in a later section.
4.2.2 Hydrogen Cyanide

Hydrogen cyanide was first detected by the Voyager 1 IRIS spectrometer through its strong infrared emission at 712 cm-1 380, and later at sub-millimeter wavelengths from ground-based observatories 381, 382, 383, 164. Although a relatively simple molecule that has been included in photochemical models for more than four decades, gaps in our knowledge of HCN formation may still exist, and new pathways have been identified recently 384.
Production: HCN is primarily produced in the upper atmosphere by the reaction of methane and nitrogen dissociation products (Fig. 25385):
| (169) | |||||
| (170) | |||||
| (171) |
and may be reformed from its ion by ion-molecule reactions, e.g.239:
| (172) |
| (173) | |||||
| (174) | |||||
| (175) |
| (176) | |||||
| (177) |
| (178) |
HCN may also be lost in a two-step process, beginning with proton-transfer from a lower proton affinity molecule, e.g.:103
| (179) |
followed by dissociative recombination:373
| (180) |
Lower in the atmosphere, radical reactions and photolysis become important:91
| (181) | |||||
| (182) |
As noted by previous authors, the CN triple bond is extremely stable and therefore the CN unit tends to persist when HCN is photolyzed, being incorporated into heavier nitriles, e.g.:
| (183) | |||||
| (184) |
Also at low altitudes ( km 103) H-addition can lead to formation of the methylene-amidogen radical:
| (185) |
Future directions: Although well-studied for decades, recent work384, 388 has identified new pathways to the formation of HCN in planetary atmospheres for which reaction rates are currently unknown. Theoretical predictions now exist, but experimental confirmation is needed.
HCN has been shown to form co-crystals with hydrocarbons at Titan-relevant temperatures 389, the study of which will be important for understanding the solids and liquids on the surface. HCN, along with HC3N, has also been implicated in the formation of C4N2 in grain-surface chemical reactions 390, which requires further study to elucidate reaction rates and whether this process is sufficient to explain observed ice spectral properties 391.
Finally, HCN has been implicated in processes of astrobiological importance. A well known example is its proposed ability to directly form the amino acid adenine (C5H5N5)392, 393, 394, 395 from the rearrangement (oligomerization) of five HCN molecules. Although the importance of this reaction for the seeding of life on the early Earth has been disputed396, 397, it may be more prevalent on Titan where HCN occurs in greater abundance.398, 399 HCN may also have the potential to polymerize into polyimines, structures that may catalyze astrobiologically important reactions 400. The astrobiological potential of HCN therefore remains under continued investigation401, 384, 402, 403.
4.2.3 Hydrogen Isocyanide

Hydrogen isocyanide, a higher energy isomer of hydrogen cyanide,404 was discovered on Titan using the Herschel space observatory by its sub-millimeter transition at 544 Ghz 405, and subsequently measured by ALMA as well 359, 128. HNC is readily interconverted to the more stable HCN (releasing kcal/mol),406 and therefore is predicted to have a steeply diminishing mixing ratio profile with altitude.98, 103
Production: HNC is produced by the same neutral reactions as HCN:
| (186) | |||||
| (187) |
where the relative productions are estimated at 1300 km.90 At 1000 km R186 becomes dominant. Note that there are two important production pathways for :
| (188) | |||||
| (189) |
with R188 dominating in the thermosphere and R189 becoming important in the mesosphere and below.90 Ion pathways may also be similar (see Fig. 26)385, although branching ratios are in most cases more uncertain than for HCN, e.g. through dissociative recombination of HCNH+:407
| (190) |
HNC may also be produced as photodissociation product of C2H3CN387 in the upper atmosphere, and a further production peak may occur due to cosmic ray chemistry at 100-150 km 98.
Loss: At high altitudes (1300 km) the principal loss channels for HNC are:90
| (191) | |||||
| (192) |
While at lower altitudes collisional isomerization to the lower energy HCN becomes important, and dominant by 600 km:90
| (193) | |||||
| (194) |
Future directions: HNC/HCN is now one of two isomer pairs known in Titan’s atmosphere (the other being ). Study of the branching ratios and reaction rates leading to and from isomer pairs/triples etc is of importance because the less stable isomer(s) may follow different reaction pathways compared to the more abundant molecule(s). Therefore for a complete understanding of Titan’s atmospheric chemistry, all isomers must be included in models. Study of the vertical ratio between HCN/HNC and CH3CCH/CH2CCH2 may also provide useful information on the abundance of atomic H, collisions which can cause conversion between the isomers.
4.2.4 Acetonitrile

Acetonitrile was first detected on Titan in the early 1990s by millimeter wavelength astronomy 408, followed ten years later by the first measurement of its vertical profile 164 using the 30 m telescope at IRAM. CH3CN was the first Titan molecule to be first detected at millimeter wavelengths, an astronomical technique that was to yield many other discoveries later with ALMA.
Production: Acetonitrile is produced in the upper atmosphere by the reaction of N-radicals with ethylene409:
| (195) | |||||
| (196) |
and by the termolecular reaction of H with cyanomethyl (CH2CN), in a chain that begins with acrylonitrile (C2H5CN):98, 103
| (197) | |||||
| (198) | |||||
| (199) |
Loss: The major loss mechanism for acetonitrile is proton transfer from another ion to form :410
| (200) | |||||
| (201) |
| (202) | |||||
| (203) |
Future directions: Acetonitrile, as many other simple molecules, has been implicated in formation of a co-crystal with acetylene 417, providing an interesting avenue for further investigation of its solid phase properties, with possible implications for cloud particle growth.
4.2.5 Cyanoacetylene

Cyanoacetylene (HC3N, propynenitrile) was first detected in Titan’s atmosphere by the Voyager IRIS spectrometer in the infrared 342 at 500 and 663 cm-1, following a prediction by Capone \latinet al.418 Cyanoacetylene, like diacetylene and cyanogen, was found in 1980 to be greatly enhanced over Titan’s northern (winter) pole - interpreted as evidence of a global stratospheric circulation cell. Gases such as HC3N with relatively short photochemical lifetimes (compared to a Titan year) have volume mixing profiles with steep vertical gradients at most latitudes, decreasing in a downwards direction as the gases become depleted and diluted. However, the presence of a strong downward motion from the mesosphere (500 km) causes enrichment in trace species to show up much lower down in the lower stratosphere (100 km).
Production: Cyanoacetylene is produced above 1000 km by reaction of the CN radical from photolysis of HCN with acetylene (see Fig. 28)202, 98, 419, 203:
| (204) |
and to a lesser extent by photodissociation of acrylonitrile (see Fig. 30).
Loss: As with other nitriles, the principal loss pathway for cyanoacetylene in the upper atmosphere is proton transfer, forming , e.g.:239
| (205) |
followed by dissociative recombination breaking up the molecule 412:
| (206) |
On the other hand, photolysis is not a significant loss channel, since is thought to rapidly recycle back to through reaction with methane:98
| (207) |
While HC3N does react without a barrier with radicals such as CN and C2H, the main loss channel in the neutral atmosphere is thought to be successive hydrogen addition:98
| (208) | |||||
| (209) | |||||
| (210) |
Future directions: In interstellar space ( molecular clouds such as TMC-1), cyanopolyynes of the form have been detected with 420, 421, 422. has been sought, but not yet detected, in Titan’s neutral atmosphere. Detection of this molecule may provide some clues as to the relative abundances of cyanopolyynes vs N-heterocycles.
4.2.6 Cyanogen

Cyanogen (C2N2), like cyanoacetylene was first detected in Titan’s atmosphere by the Voyager IRIS spectrometer in the infrared 342 at 233 cm-1.
Production: Cyanogen is thought to be produced mainly by addition of CN to HNC:
| (211) |
and through the radical-radical reaction:98
| (212) |
via the intermediate adduct NCHCN. Neither of these reactions are expected to have an entrance barrier98, 423, 424, while the reaction of CN with HCN is inefficient due to the low rate constant.425, 426, 427
Loss: Cyanogen is lost by photodissociation (Fig. 29428) and by H-addition (with an entrance barrier of 14-30 kJ/mol):98
| (213) | |||||
| (214) |
Future directions: A larger cousin to cyanogen, dicyanoacetylene () is likely to exist in Titan’s atmosphere, and detection of its ice has been proposed to explain a feature seen in Voyager IRIS and Cassini CIRS spectra at 478 cm-1, 429, 430, 391 although a lack of detection of the corresponding gas emission at 471 cm-1 has remained puzzling. Anderson \latinet al.390 have proposed a possible explanation by way of ice grain surface chemistry combining HCN and HC3N, but further laboratory and perhaps in situ experimental measurement is required to verify this hypothesis. For the time being, C2N2 remains the only dicyanide molecule known in Titan’s atmosphere.
4.2.7 Acrylonitrile
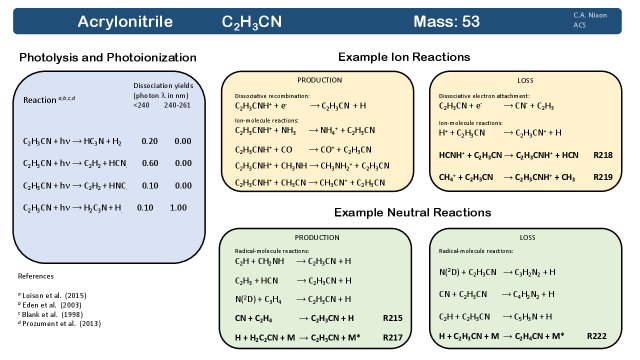
Acrylonitrile (C2H3CN) was the second molecule to be discovered on Titan at millimeter wavelengths using ALMA 166, following the detection of propionitrile,167 discussed in the next section.
Production: Acrylonitrile (see Fig. 3098, 415, 431, 432) is produced above 800 km by substitution of the CN radical onto ethylene202, 203:
| (215) |
| (216) | |||||
| (217) |
Loss: In a similar manner to HCN, and other nitriles, C2H3CN is lost in the ionosphere by the two-step process of proton transfer:433, 434
| (218) | |||||
| (219) |
followed by dissociative electron recombination:435
| (220) | |||||
| (221) |
In the lower atmosphere it may be lost to photodissociation (which tends to recycle acrylonitrile) or by H-addition:94
| (222) |
Future directions: Several small nitrile molecules, which tend to exhibit polar properties, have been investigated in a theoretical study for the potential to self-organize into spherical vesicles or membranes in non-polar liquids (such as, for example, Titan lakes and seas of methane-ethane-nitrogen). These calculations showed that acrylonitrile was the best candidate for forming so-called ‘azotosomes’ 436, which, if experimentally confirmed, could be significant for astrobiology, as vesicles (containers) for self-replicating organisms. However at this time experimental verification of azotosomes is still lacking, while a later study has questioned the ability of these structures to form 437.
4.2.8 Propionitrile

Propionitrile (C2H5CN) was the second molecule to be originally detected using sub-millimeter astronomy and the first molecule with ALMA 167.
Production: Propionitrile has been posited to be produced above 900 km 103 by the association reactions (see Fig. 31)98, 438:
| (223) | |||||
| (224) |
| (225) |
In the middle atmosphere (400–900 km) 103, successive rounds of hydrogen addition to acrylonitrile via termolecular reactions can produce propionitrile:
| (226) | |||||
| (227) |
or the termolecular reaction:98
| (228) |
Loss: As with other nitriles, the first step in loss of this nitrile in the ionosphere is proton transfer, forming :
| (229) |
This is followed by either dissociative electron recombination:440
| (230) |
or ion-neutral reactions such as:323
| (231) |
Future directions: Propionitrile has been asserted to condense in pure crystalline form in Titan’s atmosphere 441, on the basis of an unexplained feature in Titan’s far-infrared spectrum. This has been questioned based on vapor pressure of the gaseous form 442, 443, although it is possible that a co-condensed ice containing C2H5CN along with other gases may replicate the unexplained ‘haystack’ emission 444. Further work on spectroscopy will be required to determine if this is a unique solution, or if other possibilities exist.
4.2.9 Cyanopropyne

Cyanopropyne CH3C3N was the fourth molecule to be discovered by ALMA spectroscopy of Titan at mm wavelengths 168, following previous detection in the ISM 445.
Production: Production pathways for cyanopropyne (see Fig. 32) are more uncertain than for many other molecules due to the size and complexity of the molecule, allowing for more numerous reaction pathways, and multiple isomers of . Pathways involving radicals include CN substitution onto propyne or butadiene 446, 447:
| (232) | |||||
| (233) |
or attack on ethylene:448
| (234) |
| (235) | |||||
| (236) | |||||
| (237) |
Loss: Cyanopropyne is thought to be lost through either photolysis, or through protonation, e.g.:103
| (238) |
followed by dissociative electron recombination:103
| (239) | |||||
| (240) |
Future directions: , has at least three stable isomers that have been detected in space 450, 451. Besides the currently detected isomer (cyanopropyne, CH3C3N, butynenitrile or methylcyanoacetylene), there is also cyanoallene ()452 and propargyl cyanide ()453, both being first detected in the Taurus Molecular Cloud (TMC-1) at radio wavelengths. These provide good targets for detection on Titan, and their measurement would help to constrain photochemical pathways and models. Further more exotic arrangements of the same atoms may also exist and remain to be detected.
4.3 Oxygen Compounds
The oxygen chemistry of Titan’s atmosphere is apparently straightforward, with few species involved - only CO, CO2 and H2O are presently observed (see Fig. 33) - but has proven remarkably difficult to replicate in models. Early work showed difficulty in producing sufficient CO from an external flux of water (OH) 44, 46, which was originally presumed to come from meteoritic and cometary materials. The discovery of the Enceladus plumes 454, 455, 456, the connection to Saturn’s E-ring (or Enceladus torus) and subsequent finding of both OH and O+ entering Titan’s upper atmosphere 57 apparently from Enceladus, provided an abundant and unambiguous source of oxygen. Subsequent work by Hörst \latinet al.81 showed that the combination flux of O+ could finally explain the abundance of CO. In the most recent work, Vuitton \latinet al.103 have shown that OH alone is sufficient to produce the CO via previously unrecognized reaction intermediaries.

4.3.1 Water
Water was first detected in Titan’s atmosphere through infrared spectroscopy with ISO 457 through detection of emission lines at 39.4 and 43.9 m, and subsequently confirmed with Cassini CIRS 458, 459.

Production: Water (Fig. 34460, 461, 462) is thought to mainly be derived by the re-combination of OH infalling at the top of the atmosphere, primarily sourced from dissociated Enceladus water, with methane and its dissociation products :
| (241) | |||||
| (242) |
Loss: Water is lost to photolysis throughout the atmosphere, reforming hydroxyl (OH). A large fraction of this OH reacts with to reform water (see previous equation). However, OH participates in several other reactions. Above 1000 km, it reacts with to form NO:97, 373
| (243) |
while in the middle atmosphere it reacts with CO to form CO2: 97
| (244) |
| (245) | |||||
| (246) | |||||
| (247) |
and any remaining unreacted water is ultimately lost by condensation in the lower stratosphere.
Future directions: Due to its low vapor pressure, water remains difficult to measure in Titan’s atmosphere. Currently there are large uncertainties in its vertical profile 458, 459, and its latitudinal distribution remains unknown. Further work to better constrain these distributions may help to elucidate the relative importance of meteoritic versus Enceladus sources 97.
4.3.2 Carbon Monoxide

Carbon monoxide (CO, Fig. 35464) was first detected on Titan by near-IR spectroscopy, showing an absorbance of CO at 1.6 m 465, and the detection was soon confirmed at radio wavelengths 381. Estimates of its abundance fluctuated throughout the years following its discovery 466, and because these measurements were often sensitive to different altitudes, this led to the suggestion that the vertical profile was non-uniform 381, 467. Subsequent measurements with high sensitivity telescope arrays at Owens Valley and Mauna Kea however showed evidence for a uniform profile, converging on a mixing ratio of ppb 468, 469.
Recently, high-sensitivity observations with ALMA have narrowed the experimental error to a range of ppm 133, making it the fourth most abundance species in Titan’s atmosphere after N2, CH4 and H2. As a triple-bonded molecule, CO once produced is both resistant to photolysis and chemical reaction, as well having no loss through condensation, and hence all evidence available at present points to a uniform vertical profile.
Production: In the model of Vuitton \latinet al.103 CO is mostly produced via formation of formaldehyde, and subsequent photolysis:470, 471, 472
| (248) | |||||
| (249) | |||||
| (250) |
(Formaldehyde may also be created by reaction of OH with 3CH2, C2H4 etc).
| (251) | |||||
| (252) |
(also OH + 3CH2, OH + C2H4 etc). Note that formaldehyde has yet to be detected in Titan’s atmosphere.
Lesser routes to CO production may be through reaction of atomic oxygen (deposited to the top of the atmosphere from Enceladus) with methane fragments, e.g.:81
| (253) | |||||
| (254) |
Loss: CO is primarily lost slowly through reaction with OH forming CO2:474
| (255) |
which is in turn lost through condensation.
Future directions: Laboratory chemistry simulations of Titan’s atmosphere have shown that CO may react with methane and nitrogen when sufficiently stimulated, forming amino acids and even nucleobases 475. This provides an exciting possibility of astrobiology that now requires remote and in situ measurements to confirm.
4.3.3 Carbon Dioxide

Carbon dioxide (CO2) was one of seven new gas species detected by Voyager’s IRIS infrared spectrometer 476, 477 and subsequently by Cassini CIRS 478, 278. Unlike shorter-lived chemical chemical species ( HC3N, C4H2), CO2 exhibits little variation with latitude in the lower stratosphere, lacking a polar enhancement.
Production: CO2 is thought to be mostly produced from CO + OH as shown in the previous section (R 255). See also Fig. 36464, 479, 103.
| (257) | |||||
and also through condensation in the lower stratosphere.
4.4 Growth of large particles

As hydrocarbon molecules grow to ever-larger sizes, they may take several forms: long chains, fused rings (PAHs - polycyclic aromatic hydrocarbons), or rings connected by hydrogen bonds (poly-phenyls) (see Fig. 37). Nitrogen incorporation is also likely, for example in the form of PANHs (polycyclic aromatic nitrogen heterocycles). It is thought that eventually larger molecules clump together due to electrostatic forces to form fractal aggegrates 486, 212, 487, 488 - Titan haze particles which form the well-known golden haze at visible wavelengths. These in turn become the nuclei for stratospheric hydrocarbon ice particles or tropospheric methane raindrops 114, 115, 116, 117, and fall to the surface where they form Titan’s dune fields 118.

The presence of PAHs on Titan has been studied as far back as the 1990s 489 in laboratory experiments. However despite these predictions, detection of specific PAHs has remained elusive. The closest we have come so far to identification of a unique PAH in Titan’s atmosphere was the sighting of a peak at in the CAPS spectrum by Waite \latinet al.61, along with another peak at twice the mass: . These were tentatively identified as due to anthracene and its dimer (Fig. 38), although non-aromatic structures could not be ruled out. Note that the dimer itself may have formed by wall reactions in the instrument, but this would not rule out the fact that anthracene was entering the instrument. Likewise, no polyphenyls or N-heterocycles have been uniquely identified.
Production: The formation of PAHs by addition to benzene rings has been a topic of debate for considerable time. Recently, Kaiser and Hansen353 have categorized possible pathways into five principal mechanisms:
-
•
HACA - "hydrogen abstraction- C2H2 addition"490
-
•
HAVA - "hydrogen abstraction- vinylacetylene addition"
-
•
PAC - "phenyl addition-dehydrocyclization"
-
•
RRR - "radical-radical reactions"
-
•
MACA - "methylidene addition-cyclization aromatization"
Each of these offers potential pathways to larger molecules. In brief, the HAVA mechanism, in which vinylacetylene () adds to aromatic rings (such as benzene) in a barrierless reaction is thought to be principle mechanism by which additional six-membered rings are added to existing rings at low temperatures, such as in planetary atmospheres. The other mechanisms offer alternate routes to addition of five and six-sided rings, predominantly at high temperatures of 1000s of K (HAVA, PAC, RRR), although MACA may operate at low temperatures to form indene. The reader is directed to the review paper by Kaiser and Hansen353 for a full description, which is beyond the scope of this article.
No discussion of aerosol particle growth would be complete without mention of negative ions. The discovery of large negatively charged ions at high altitudes by Cassini’s CAPS instrument was one of the major surprises about Titan’s atmosphere early in the mission 61, 60, 69, 491, 492, 68. Small negative ions may be formed by several processes, including dissociative electron attachment, e.g.:
| (258) | |||||
| (259) |
and by radiative attachment to a radical species already formed through photochemistry:103
| (260) | |||||
| (261) | |||||
| (262) | |||||
| (263) |
Once H- is produced, it leads to the creation of some larger negative ions through proton abstraction:103
| (264) | |||||
| (265) |
Successively larger aerosol particles are produced through a variety of ion-neutral and ion-ion reactions 493, 494, 495. The largest charged particles tend to be predominantly negative ions, due to the larger to their higher mobility.
Loss: Since aromatic rings, once formed, are very stable, with many possibilities to disperse absorbed energy internally, their principle loss channels will be either (a) to form radicals and then larger molecules; (b) to agglomerate or (c) to condense.
Future directions: Much work is still required to further elucidate the growth of large particles, especially the relative important of ion vs neutral chemistry at different altitudes. See also the later Section on the topic of PAHs.
5 Gaps in our knowledge
In this section we consider where the gaps are in our current knowledge and understanding of Titan’s atmospheric composition and chemistry, and how these gaps might be addressed in the near future through combinations of astronomy, laboratory and theoretical work.
5.1 Aliphatic Species
5.1.1 Hydrocarbons
At present, C4H2 remains the only hydrocarbon species definitively detected in the neutral atmosphere, while no aliphatic species with have been detected (benzene, c-C6H6, being detected as a ring).
| (266) | |||||
| (267) | |||||
| (268) |
Species with four or more carbon atoms show increased possibilities for structural isomerization, as illustrated in Fig. 39. This results in both increased challenges for observational detection, as well as new and interesting chemical possibilities such as branched chain molecules.
Upper limits for -butane and -butane (C4H10) have been calculated as and respectively, from CIRS spectra at 200–250 km, 30∘N–30∘S498.
Future Work: Astronomical detection of species such as , (i.e. butynes and butenes) and even would greatly help to improve constraint on photochemical models, which are currently lacking in data to model for this regime. Building our knowledge of aliphatic species such as C4’s and C5’s may provide a better understanding of the pathways to benzene or other cyclic species.
In parallel, photochemical models that currently treat multiple isomers under single formulae such as and must continue to expand treatment of separate isomers. To date this has been sparse and primarily for a few specific cases for which isomeric data exists: especially HCN and HNC, and CH2CCH2 and CH3CCH, which are considered separately in current models 90, 91, 99, 103. A good reason for lack of inclusion of separate isomers is lack of knowledge of branching ratios and isomer-specific reaction rates. Monte Carlo simulations have proved useful at showing which reaction rate uncertainties have the biggest effects on the uncertainties in the solution 87, 88. These researches thereby provide important prioritization of where resources such as laboratory time and theoretical effort (e.g. TST calculations) can best be spent to most rapidly improve our knowledge.
5.1.2 Nitrogen Species
All of the nine detected nitrogen compounds in Titan’s neutral atmosphere, formed from dissociation products of N2 and CH4, are triple bonded. These include N2 itself, and eight known cyanides, which include nitrogen in a terminal -CN functional group.
Nitriles (cyanides) appear stable and plentiful in Titan’s atmosphere, and further examples are sure to be found. In 1985 in the wake of the Voyager IRIS discoveries, Cerceau \latinet al.499 studied the infrared spectra of seven undetected nitriles to facilitate further new detections. 35 years later, four of these seven species had been detected on Titan (CH3CN, C2H3CN, C2H5CN and CH3C3N), although ironically all these detections were made through sub-millimeter wave astronomy, not infrared spectroscopy 164, 167, 166, 168. Upper limits for the three remaining undetected species, plus one other have been calculated by Coustenis \latinet al.500:
| crotonitrile | () | |
| butanenitrile | () | |
| isobutyronitrile | () | |
| cyanocycloproane | () | . |
Nitrogen has yet to be detected in other types of bonding - i.e. where it is not in a triple bond, such as in amines, imines (beyond HNC), azines and nitrogen heterocycles (see Fig. 40). A simple example is ammonia (NH3) which was tentatively inferred from Cassini’s mass spectra at high altitudes, at mixing fractions of , although not yet uniquely detected due to the barometric degeneracy problem of unit resolution mass spectroscopy.501, 219
The major channel for ammonia production is thought to be via NH2 + CH2N, as follows:103
| (269) | |||||
| (270) | |||||
| (271) | |||||
| (272) |
Ammonia is mostly lost through photodissociation.
Upper limits for some molecules in the lower atmosphere have been estimated, including for ammonia (NH3) of 1.3 ppb (3-) at 107 km, 25∘S 502, and methanimine (CH2NH) at 0.35 ppb (3-) in the stratosphere 503.

Future Work: No molecules having both oxygen and nitrogen, e.g. HNO, and more complex amino acids, have been detected. Detection of such functional groups and molecules, important for biological activity on Earth, is a key area of future research. Several nitriles have been detected only through sub-millimeter astronomical techniques as previously mentioned, but not using infrared techniques despite several decades of attempts 499, 500, 49, 502. In fact laboratory line lists with intensities do not exist for many of the nitriles sought in the infrared, except CH3CN 504, 505, hampering the search and implying a need for new laboratory work to obtain cold temperature spectra.
5.1.3 Oxygen species
Few oxygen compounds have been detected in Titan’s atmosphere: only CO, CO2 and H2O to date. Diatomic molecular oxygen, , readily found in the atmospheres of the inner planets, is absent, allowing organic chemistry to proceed to great complexity. The oxygen compounds detected appear to be attributable to an external source of oxygen (both O and OH), most likely originating from Enceladus 57, 81. Prior to the discover of the Enceladus plumes a meteoritic source was favored 506, and some meteoritic contribution may still be present 97.
Other than CO, which is present at a relatively high abundance ( ppm 133), oxygen is a minor although potentially important ingredient of Titan’s atmosphere. This is because many molecules of biological importance require oxygen 507. As early as the 1980s it had already been demonstrated that hydrolysis of Titan tholins () added oxygen to form amino acids of biological relevance 508 - subsequently confirmed in many similar experiments. More recently, Hörst \latinet al.475 showed that even in the gas phase, amino acids may be synthesized in a Titan-like atmosphere when CO is added to mixtures of CH4 and N2 in RF discharge experiments.
Photochemical models 97 predict that trace amounts of molecules such as HNO, HNCO, H2CO and CH3OH should be present in the atmosphere, and perhaps detectable at high altitudes by observatories such as ALMA. To date, few published studies have attempted to directly identify further oxygen species. The Cassini INMS team published upper limits for methanol () and acetaldehyde () of 30 ppb and 10 ppb in the ionosphere at 1100 km 219. In the stratosphere, upper limits have been determined for methanol and formaldehyde of 6 ppb and 2 ppb respectively at at 107 km, 25∘S 502 from infrared spectroscopy with Cassini CIRS.
Future Work: Vibrational (IR) and/or rotational (sub-mm) line lists exist for most small oxygen compounds currently undetected in Titan’s atmosphere (see Fig. 41), making astronomical searches viable.509, 510, 511

5.2 Cyclic molecules
5.2.1 Single hydrocarbon rings
Two small cyclic molecules have been definitively detected in Titan’s neutral atmosphere: cyclopropenylidene (c-C3H2) 165 and benzene (c-C6H6) 49. Other small cyclic molecules likely to exist include the saturated cycloalkanes - cyclopropane (), cyclobutane () and larger - and possibly cycloalkenes such as cyclopropene (), cyclobutene (), cyclobutadiene (), and others. Substituted rings are also possible: cyanocyclopropane ().
In the ISM, several single ring molecules have been detected. These include the small 3-carbon rings cyclopropenylidene (c-C3H2 306) and its related radical 512, and a substituted species, ethynyl cyclopropenylidene () 513. The five-sided ring cyclopentadiene (), and the six-sided rings benzyne () and benzene (c-C6H6) have also been detected 348, 513, 514.
Future work: Work is needed on all fronts to advance our understanding of the formation pathways, stability and prevalence of small cyclic rings in Titan’s atmosphere (see Fig. 42). These include astronomical observations, laboratory work and photochemical modeling. In the 2030s, we anticipate direct in situ measurement of such molecules by the Dragonfly probe DraMS (Dragonfly Mass Spectrometer) instrument, which, unlike Cassini INMS will have ability to definitively identify molecular structure through a combination of tandem mass spectrometry (MS/MS) and Gas Chromatograph Mass Spectrometry (GCMS) 515.

5.2.2 Multi-ring hydrocarbons: PAHs and polyphenyls
Polycyclic aromatic hydrocarbons (PAHs) are multi-ring molecules composed of carbon and hydrogen that exhibit aromatic character, that is to say they have delocalized electron bonds. Example include naphthalene (two 6-membered rings), indene (one five-sided and one six-sided ring), anthracene and phenanthrene (three 6-membered rings), and larger examples (see Fig. 43). Benzene is not considered to be a PAH, since it has only one ring.

PAHs have long been suspected to exist in interstellar space516, and have been implicated as culprits responsible for the so-called ‘diffuse interstellar bands’ (DIBs)517 (see review by 518). To date, only a single non-functionalized PAH has been uniquely identified in space (indene - 513), although a greater number of CN-substituted single and double rings (cyano-PAHs) have been identified, assisted by their strong rotational lines due to the cyanide group - see review by McCarthy and McGuire519.
Near-IR emission at 3.28 m has also been seen in Titan’s dayside spectrum by Cassini VIMS 520. In a model by Lopez-Puertas \latinet al.521 this emission was attributed to a combination of PAHs with 9-96 carbons (up 11 fused rings), using laboratory cross-section as measured in the Ames PAH database 522, however unique identification of individual PAHs was not possible.
Laboratory experimental work on haze formation by UV photolysis has shown that the inclusion of benzene in initial reagent mixtures along with N2 and CH4 leads to the formation of significantly larger molecules than when a N2/CH4 mixture is used 523. More recently, naphthalene has also be used as a starting reagent in lab tholin experiments 524 showing similar results. It should be noted that, on Titan, both benzene and naphthalene would presumably first have to form from methane, so their use in lab experiments may be considered an acceleration of a natural process that could in principle start from a pure N2/CH4 mixture and arrive at the same result over long time periods.
Multi-ring organic molecules are not constrained to form as ‘fused’ ring PAHs such as naphthalene, but may instead form as polyphenyls (see Fig. 37). It has been argued that a significant amount of carbon rings in Titan’s atmosphere may be in the form of polyphenyls rather than as fused rings 525. Titan’s aerosols are likely to be a mixture of fused and unfused rings, forming monomers and then fractal aggregates 526.
Future work: Future laboratory, and eventual in situ experimental work is required to determine the relative importance of fused vs non-fused rings.
5.2.3 Fullerenes

Fullerenes are carbon allotropes formed of rings of 5 to 7 atoms in closed or partially closed mesh structures. These can include ‘buckyballs’ (or buckminsterfullerenes), such as the spherical () and ellipsoidal () molecules 527, 528, but also as sheets (graphene) and cylinders (carbon nanotubes) (see Fig. 44). Despite their large size and apparent complexity, buckyballs have been detected in space both as neutrals 529 and ions 530, and in meteorites 531. Fullerenes have been hypothesized to exist in Titan’s atmosphere 532 although a recent attempt to detect them in Spitzer data proved unsuccessful.533
Future work: Greater sensitivity with observatories such as JWST 534 may enable more sensitive searches for fullerenes in Titan’s atmosphere. In addition, little lab work has been done at present to determine what effect the presence of fullerenes could have on Titan’s atmospheric chemistry, aerosol formation and surface geology.
5.2.4 PANHs

Nitrogen heterocycles and PANHs (polycyclic aromatic nitrogen heterocycles) are similar to PAHs, but with nitrogen incorporation into the ring structure (see Fig. 45, 165). Nitrogen-heterocycles have been sought unsuccessfully in interstellar space, with upper limits for molecules such as pyridine and quinoline derived 535. Mass spectroscopy of Titan’s atmosphere with Cassini INMS has identified peaks at masses that could correspond to N-heterocycles, such as (could be protonated pyridine) at mass 80 and at mass 81 (possibly protonated pyrimidine) 219. However, aliphatic variants are possible making the PANH ion identification uncertain.
Recently, Nixon \latinet al.165 made the first astronomical search for pyridine and pyrimidine in Titan’s atmosphere using ALMA, deriving upper limits on their disk-averaged (global) abundances: pyridine (c-C5H5N) at 1.15 ppb (2-) above 300 km 165; and similarly pyrimidine (c-C4H4N2) at 0.85 ppb (2-) also above 300 km.
Laboratory work has examined how tholin (Titan haze analog) formation in UV photolysis experiments is affected by the inclusion of N-heterocycles such as pyridine and quinoline 536, 537. Changes in the spectrum show similarities to features in Titan’s haze spectrum 536, and the structures formed show a mixture of polymeric and random co-polymeric structure 524.
Future work: In future, more sensitive astronomical searches may be undertaken, for example using ALMA and JWST. Photochemical models at present do not include detailed description of N-heterocycle formation and growth, which must be included in future generations to adequately model processes leading to haze formation.
5.3 Sulfur and Phosphorous Chemistry
To date, no compounds of sulfur or phosphorus have been found in Titan’s atmosphere. Nixon \latinet al.538 made the first quantitative assessment of upper limits for the most simple reduced species - PH3 and H2S - of 1 ppb and 300 ppb respectively in the stratosphere (250 km). Phosphine gas has even been mooted as a biosignature gas in planetary atmospheres 539, although this conclusion has been debated.540 A sensitive search for CS was made by Teanby \latinet al.503 with ALMA, yielding an upper limit of either 7.4 ppt (uniform profile above 100 km) or 25.6 ppt (uniform profile above 200 km).
The first (and only so far) photochemical model to include sulfur chemistry was published by Hickson \latinet al.541, which predicts that CS and H2CS should be the most abundant sulfur-bearing species in the upper atmosphere, transitioning to C3S, H2S and CH3SH in the lower atmosphere. However in the absence of constraints, predictions remain highly uncertain.
In principle the O/S ratio should allow further constraint of the source of Titan’s oxygen flux, since the O/S ratio is predicted to be some 1000 less for a cometary source (O/S100)542 than an Enceladus source (O/S)543. Detection of sulfur in Titan’s atmosphere may also provide evidence for cryovolcanic activity 252.
Both phosphorus and sulfur are among the six most essential elements for biochemistry on Earth (the so-called CHNOPS elements). With four of the six already detected on Titan, it is therefore of considerable interest to seek the remaining two, to further assess Titan’s potential for astrobiology. Recent reports that all six CHNOPS elements have now been detected in the plume material of Enceladus 543, 544, make it feasible that some trace amounts of P and S arrive at the top of Titan’s atmosphere, as is the case apparently with O57.
Future work: Future work is required on both the direct detection of P and S-containing substances by both astronomical and in-situ techniques - but also in laboratory work, computer photochemical modeling, and clarification of reaction pathways and rates, especially at low temperatures.
5.4 Radicals
For completeness, we include consideration of radical species - atoms and molecules with unpaired free electrons - even though these are highly reactive and unlikely to be found in significant numbers, or significantly deep in Titan’s neutral atmosphere. Radicals include fragments of CH4, such as CH (methylidene), CH2 (methylene), and CH3 (methyl), as well as ground state and excited nitrogen atoms formed from break up of N2, such as as N(2D) and N(4S).
Many radicals (CN, OH etc) have been observed in the ISM 450, including at least one cyclic radical ()512; also in comets 545 and tenuous satellite exospheres 546. However, to date only the methyl radical has been detected by astronomical techniques in a planetary atmosphere 547, 548.
Future Work: ALMA observations are particularly sensitive to Titan’s upper atmosphere, and can sense HCN at altitudes of up to 1200 km 128, 159. Future investigations with ALMA may allow the detection of radicals with dipoles, and more sensitive infrared observatories such as JWST may prove effective at detecting radicals such as methyl in the infrared 534.
6 Conclusions
The organic-rich atmosphere of Titan constitutes the most complex atmospheric chemical network known outside of Earth. This provides a unique natural laboratory for understanding the synthesis of organic compounds, processes that may have been important early in the history of the Solar System 549, 550, and may have seeded the origins of life on Earth 551, 552.
Therefore it is of substantial scientific importance to better understand these processes and chemical results. This field of enquiry brings together astronomers, laboratory chemists, theoretical chemists and atmospheric modelers whose combined approaches are needed to unravel the entire picture. Substantial progress has been made, especially stimulated by the recent wealth of data from the Cassini-Huygens mission 2, 3, and the selection of the Dragonfly mission275 that will arrive in the 2030s.
Astronomy: Titan is a distant object and gathering robust information is difficult in the absence of spacecraft: astronomy is currently the only means to gather data about Titan directly. Currently active ground and space-based observatories such ALMA, IRTF and JWST are providing continuity of data collection since the end of Cassini-Huygens regarding seasonal changes in Titan’s atmosphere,159, 147 and new information on the chemistry, composition and isotopic ratios.170, 171, 325, 553, 166, 168, 165, 554, 133.
Laboratory Studies: Whilst data collection through astronomy and remote sensing continues, a robust campaign of laboratory experiments and theoretical work is continuing in parallel to understand the origins and chemical evolution of the atmosphere, and interaction with the surface and subsurface. These diverse enquiries include spectroscopy of gases,555, 341, 556, 557, 558, 559, 560, 498, 561, 562, 563 measurement of reaction rates,358, 347, 564, 565, 409, 242, 566, 567, 354, 568 experimental work on co-crystals,297, 276, 277, 417, 569, 570 ices,571, 572, 573, 574, 575, 576, 577, 578, 579, 580, 581, 582 and hazes (tholins).111, 110, 583, 584, 585, 586, 587, 588, 589, 590, 591, 592
Modeling: The chemistry of Titan’s atmosphere cannot proceed without disturbing the medium in which it takes place: the minor gases and haze generated have a substantial effect on the heating and cooling of the atmosphere 593, and in turn changes to the thermal structure of the atmosphere leads to dynamical motions including vertical eddy mixing, and meridional transport. As gases are transported, they enter atmospheric regions that may have greater or lesser photon flux from the Sun, have fewer or greater opportunities to interact with electrons, and encounter differing densities of radicals and other reagents. Therefore, decoupling chemistry from dynamics is not possible. Combining the current generation of Global Circulation Models (GCMs) 594, 595, 155, 596, 597, 598 and photochemical models 99, 100, 102, 103, 599 to create 2D and 3D coupled chemical-GCMs is a challenging but important task that must occupy the next generation of modelers.
Future Missions: The Dragonfly mission estimated to land on Titan 2034 will provide a wealth of new data about Titan’s surface and atmospheric boundary layer. However Dragonfly will only investigate the low latitudes, including dunes and the crater Selk. In the future an orbiter, as envisaged by several published studies 600, 601, 602, would provide the important benefit of a truly global picture, including potentially complete global mapping of the atmosphere and surface at uniform resolution, with time-domain information to search for changes occurring. Other elements such as a balloon, airplane and/or floating lake probe could provide valuable in situ information about other environs 603, 604, 605.
There is no doubt that Titan still offers many challenges to our understanding that will provide fertile areas of study for future generations of scientists 21, 606, and offer rewards through important insights into the chemical evolution of the Solar System and origins of life in the universe.
Funding for this work was through NASA’s Astrobiology program. The author wishes to thank the Astrobiology Program manager Dr Mary Voytek (NASA HQ) and the Principal Investigator of the CAN-8 Project "Habitability of Hydrocarbon Worlds: Titan and Beyond" Dr Rosaly Lopes (JPL/Caltech) for their support of this work. Thanks is also due to Nicholas Lombardo and Alexander Thelen for providing text files of retrieved gas profiles from prior publications. The author sincerely thanks two anonymous reviewers and the guest editors of the ACS Earth and Space Chemistry special edition on astrochemistry, Martin Cordiner and Christopher Bennett for their thoughtful comments and feedback that helped to improve the manuscript; and to the Journal Editor Eric Herbst, and staff for their assistance in the review and publication process. Last but not least, the author is very grateful to a cadre of early-career students and postdocs who proof-read parts of the final submitted version of the manuscript: Brandon Coy (UCLA), Nicholas Kutsop (Cornell University), Paige Leeseberg (University of Iowa/SURA), Siobhan Light (University of Maryland/SURA), Nicholas Lombardo (Yale University), Edward Molter (University of California Berkeley), Jonathon Nosowitz (Catholic University), Alexander Thelen (Caltech). Any remaining errors or inaccuracies are the responsibility of the author.
Appendix A Acronyms and Abbreviations
| ALMA | Atacama Large Millimeter/submillimeter Array |
|---|---|
| CAPS | Cassini Plasma Spectrometer |
| CDA | Cosmic Dust Analyzer (Cassini instrument) |
| CIRS | Composite Infrared Spectrometer (Cassini instrument) |
| DR | Dissociative Recombination |
| DSMC | Direct Simulation Monta Carlo |
| ESA | European Space Agency |
| GCM | Global Circulation Model |
| GCMS | Gas Chromatograph / Mass Spectrometer (Huygens instrument) |
| HASI | Huygens Atmospheric Structure Instrument |
| INMS | Ion and Neutral Mass Spectrometer (Cassini instrument) |
| IRAM | Institut de Radioastronomie Millimetrique |
| IRIS | Infrared Interferometer Spectrometer (Voyager instrument) |
| IRTF | Infrared Telescope Facility |
| ISM | InterStellar Medium |
| ISO | Infrared Space Observatory |
| IUPAC | International Union of Pure and Applied Chemists |
| JWST | James Webb Space Telescope |
| LTE | Local Thermodynamic Equilibrium |
| NASA | National Aeronautics and Space Administration |
| PAH | Poly-Aromatic Hydrocarbon |
| PANH | Poly-Aromatic Nitrogen Heterocycle |
| RPWS | Radio and Plasma Wave Spectrometer (Cassini instrument) |
| RSS | Radio Science Subsystem (Cassini| instrument) |
| SNR | Signal to Noise Ratio |
| TEXES | Texas Echelon Cross Echelle Spectrograph |
| TMC | Taurus Molecular Cloud |
| TST | Transition State Theory |
| UVIS | Ultraviolet Imaging Spectrometer (Cassini instrument) |
| VIMS | Visible and Infrared Mapping Spectrometer (Cassini instrument) |
| VMR | Volume Mixing Ratio |
References
- Lebreton \latinet al. 2005 Lebreton, J.-P.; Witasse, O.; Sollazzo, C.; Blancquaert, T.; Couzin, P.; Schipper, A.-M.; Jones, J. B.; Matson, D. L.; Gurvits, L. I.; Atkinson, D. H.; et al., An overview of the descent and landing of the Huygens probe on Titan. Nature 2005, 438, 758–764
- Lebreton 2002 Lebreton, J.-P. The Huygens Probe: Science, Payload and Mission Overview. Space Science Reviews 2002, 104, 59–100
- Matson 2002 Matson, D. L. The Cassini/Huygens Mission to the Saturnian System. Space Science Reviews 2002, 104, 1–58
- Hueso and Sánchez-Lavega 2006 Hueso, R.; Sánchez-Lavega, A. Methane storms on Saturn’s moon Titan. Nature 2006, 442, 428–431
- Lunine and Atreya 2008 Lunine, J. I.; Atreya, S. K. The methane cycle on Titan. Nature Geoscience 2008, 1, 159–164
- Witek and Czechowski 2015 Witek, P. P.; Czechowski, L. Dynamical modelling of river deltas on Titan and Earth. Planetary and Space Science 2015, 105, 65–79
- Hayes 2016 Hayes, A. G. The Lakes and Seas of Titan. Annual Review of Earth and Planetary Sciences 2016, 44, 57–83
- Kasting 1993 Kasting, J. F. Earth’s early atmosphere. Science 1993, 259, 920–926
- McKay \latinet al. 1999 McKay, C. P.; Lorenz, R. D.; Lunine, J. I. Analytic Solutions for the Antigreenhouse Effect: Titan and the Early Earth. Icarus 1999, 137, 56–61
- Tian \latinet al. 2005 Tian, F.; Toon, O. B.; Pavlov, A. A.; De Sterck, H. A hydrogen-rich early Earth atmosphere. Science 2005, 308, 1014–1017
- Trainer \latinet al. 2006 Trainer, M.; Pavlov, A.; DeWitt, H.; Jimenez, J.; McKay, C.; Toon, O.; Tolbert, M. Organic haze on Titan and the early Earth. PNAS; Proceedings of the National Academy of Sciences 2006, 103, 18035–18042
- He and Smith 2014 He, C.; Smith, M. A. Identification of nitrogenous organic species in Titan aerosols analogs: Implication for prebiotic chemistry on Titan and early Earth. Icarus 2014, 238, 86–92
- Zahnle \latinet al. 2020 Zahnle, K. J.; Lupu, R.; Catling, D. C.; Wogan, N. Creation and evolution of impact-generated reduced atmospheres of early Earth. The Planetary Science Journal 2020, 1, 11
- Sessions \latinet al. 2009 Sessions, A. L.; Doughty, D. M.; Welander, P. V.; Summons, R. E.; Newman, D. K. The Continuing Puzzle of the Great Oxidation Event. Current Biology 2009, 19, R567–R574
- Gumsley \latinet al. 2017 Gumsley, A.; Chamberlain, K.; Bleeker, W.; Söderlund, U.; de Kock, M.; Larsson, E.; Bekker, A. Timing and tempo of the Great Oxidation Event. PNAS; Proceedings of the National Academy of Sciences 2017, 114, 1811–1816
- Zahnle and Carlson 2020 Zahnle, K. J.; Carlson, R. W. Creation of a habitable planet. Planetary astrobiology 2020, 3–37
- Lunine \latinet al. 2010 Lunine, J.; Choukroun, M.; Stevenson, D.; Tobie, G. In Titan from Cassini-Huygens; Brown, R. H. and Lebreton, J.-P. and Waite, J. H.,, Ed.; 2010; pp 35–59
- Bourgalais \latinet al. 2021 Bourgalais, J.; Carrasco, N.; Miguel, Y.; Venot, O.; Pernot, P. Ion-driven organic chemistry for Titan-like atmospheres: Implications for N-dominated super-Earth exoplanets. Astronomy & Astrophysics 2021, 654, A171
- Woitke \latinet al. 2021 Woitke, P.; Herbort, O.; Helling, C.; Stüeken, E.; Dominik, M.; Barth, P.; Samra, D. Coexistence of CH4, CO2, and H2O in exoplanet atmospheres. Astronomy & Astrophysics 2021, 646, A43
- Hörst 2017 Hörst, S. M. Titan’s atmosphere and climate. Journal of Geophysical Research: Planets 2017, 122, 432–482
- Nixon \latinet al. 2018 Nixon, C.; Lorenz, R.; Achterberg, R.; Buch, A.; Coll, P.; Clark, R.; Courtin, R.; Hayes, A.; Iess, L.; Johnson, R.; et al., Titan’s cold case files - Outstanding questions after Cassini-Huygens. Planetary and Space Science 2018, 155, 50–72
- Brown \latinet al. 2010 Brown, R. H., Lebreton, J.-P., Waite, J. H., Eds. Titan from Cassini-Huygens; Springer Netherlands, 2010
- Muller-Wodarg \latinet al. 2014 Muller-Wodarg, I., Griffith, C. A., Lellouch, E., Cravens, T. E., Eds. Titan; Cambridge University Press, 2014
- Niemann \latinet al. 2002 Niemann, H. B. \latinet al. The Gas Chromatograph Mass Spectrometer for the Huygens Probe. Space Science Reviews 2002, 104, 553–591
- Brown \latinet al. 2004 Brown, R. H. \latinet al. The Cassini Visual And Infrared Mapping Spectrometer (VIMS) Investigation. Space Sci. Rev. 2004, 115, 111–168
- Esposito \latinet al. 2004 Esposito, L. W.; Barth, C. A.; Colwell, J. E.; Lawrence, G. M.; McClintock, W. E.; Stewart, A. I. F.; Keller, H. U.; Korth, A.; Lauche, H.; Festou, M. C.; et al., The Cassini Ultraviolet Imaging Spectrograph Investigation. Space Science Reviews 2004, 115, 299–361
- Waite \latinet al. 2004 Waite, J. H. \latinet al. The Cassini Ion and Neutral Mass Spectrometer (INMS) Investigation. Space Sci. Rev. 2004, 114, 113–231
- Waite \latinet al. 2005 Waite, J. H. \latinet al. Ion Neutral Mass Spectrometer Results from the First Flyby of Titan. Science 2005, 308, 982–986
- Shemansky \latinet al. 2005 Shemansky, D. E.; Stewart, A. I. F.; West, R. A.; Esposito, L. W.; Hallet, J. T.; Liu, X. The Cassini UVIS stellar probe of the Titan atmosphere : Cassini reveals Titan. Science 2005, 308, 5
- Bellucci \latinet al. 2009 Bellucci, A.; Sicardy, B.; Drossart, P.; Rannou, P.; Nicholson, P.; Hedman, M.; Baines, K.; Burrati, B. Titan solar occultation observed by Cassini/VIMS: Gas absorption and constraints on aerosol composition. Icarus 2009, 201, 198–216
- Niemann \latinet al. 2010 Niemann, H. B.; Atreya, S. K.; Demick, J. E.; Gautier, D.; Haberman, J. A.; Harpold, D. N.; Kasprzak, W. T.; Lunine, J. I.; Owen, T. C.; Raulin, F. Composition of Titan’s lower atmosphere and simple surface volatiles as measured by the Cassini-Huygens probe gas chromatograph mass spectrometer experiment. Journal of Geophysical Research (Planets) 2010, 115, 12006–+
- Niemann \latinet al. 2005 Niemann, H. B. \latinet al. The abundances of constituents of Titan’s atmosphere from the GCMS instrument on the Huygens probe. Nature 2005, 438, 779–784
- Khare \latinet al. 1986 Khare, B. N.; Sagan, C.; Ogino, H.; Nagy, B.; Er, C.; Schram, K. H.; Arakawa, E. T. Amino acids derived from Titan Tholins. Icarus 1986, 68, 176–184
- Neish \latinet al. 2010 Neish, C. D.; Somogyi, A.; Smith, M. A. Titan’s primordial soup: formation of amino acids via low-temperature hydrolysis of tholins. Astrobiology 2010, 10, 337–347
- Ramírez \latinet al. 2010 Ramírez, S.; Coll, P.; Buch, A.; Brassé, C.; Poch, O.; Raulin, F. The fate of aerosols on the surface of Titan. Faraday discussions 2010, 147, 419–427
- Raulin 2008 Raulin, F. Astrobiology and habitability of Titan. Strategies of life detection 2008, 37–48
- Raulin \latinet al. 2010 Raulin, F.; McKay, C.; Lunine, J.; Owen, T. Titan’s astrobiology. Titan from Cassini-Huygens 2010, 215–233
- Lunine \latinet al. 2020 Lunine, J. I.; Cable, M. L.; Hörst, S. M.; Rahm, M. The astrobiology of Titan. Planetary astrobiology 2020, 247
- Strobel 1974 Strobel, D. F. The photochemistry of hydrocarbons in the atmosphere of Titan. Icarus 1974, 21, 466–470
- Strobel 1982 Strobel, D. Chemistry and evolution of Titan’s atmosphere. Planetary and Space Science 1982, 30, 839–848
- Yung \latinet al. 1984 Yung, Y. L.; Allen, M.; Pinto, J. P. Photochemistry of the atmosphere of Titan - Comparison between model and observations. Astrophys. J. Supp. 1984, 55, 465–506
- Yung 1987 Yung, Y. L. An update of nitrile photochemistry on Titan. Icarus 1987, 72, 468–472
- Toublanc \latinet al. 1995 Toublanc, D.; Parisot, J.; Brillet, J.; Gautier, D.; Raulin, F.; McKay, C. Photochemical Modeling of Titan’s Atmosphere. Icarus 1995, 113, 2–26
- English \latinet al. 1996 English, M.; Lara, L.; Lorenz, R.; Ratcliff, P.; Rodrigo, R. Ablation and chemistry of meteoric materials in the atmosphere of Titan. Advances in Space Research 1996, 17, 157–160
- Lara \latinet al. 1994 Lara, L.; Lorenz, R.; Rodrigo, R. Liquids and solids on the surface of Titan: results of a new photochemical model. Planetary and Space Science 1994, 42, 5–14
- Lara \latinet al. 1996 Lara, L. M.; Lellouch, E.; López-Moreno, J. J.; Rodrigo, R. Vertical distribution of Titan’s atmospheric neutral constituents. Journal of Geophysical Research: Planets 1996, 101, 23261–23283
- Wilson and Atreya 2004 Wilson, E. H.; Atreya, S. K. Current state of modeling the photochemistry of Titan’s mutually dependent atmosphere and ionosphere. Journal of Geophysical Research (Planets) 2004, 109, 6002–+
- Coustenis \latinet al. 1989 Coustenis, A.; Bézard, B.; Gautier, D. Titan’s atmosphere from Voyager infrared observations: I. The gas composition of Titan’s equatorial region. Icarus 1989, 80, 54–76
- Coustenis \latinet al. 2003 Coustenis, A.; Salama, A.; Schulz, B.; Ott, S.; Lellouch, E.; Encrenaz, T. H.; Gautier, D.; Feuchtgruber, H. Titan’s atmosphere from ISO mid-infrared spectroscopy. Icarus 2003, 161, 383–403
- Keller \latinet al. 1992 Keller, C.; Cravens, T.; Gan, L. A model of the ionosphere of Titan. Journal of Geophysical Research: Space Physics 1992, 97, 12117–12135
- Fox and Yelle 1997 Fox, J. L.; Yelle, R. V. Hydrocarbon ions in the ionosphere of Titan. Geophysical research letters 1997, 24, 2179–2182
- Galand \latinet al. 1999 Galand, M.; Lilensten, J.; Toublanc, D.; Maurice, S. The Ionosphere of Titan: Ideal Diurnal and Nocturnal Cases. Icarus 1999, 140, 92–105
- Müller-Wodarg \latinet al. 2000 Müller-Wodarg, I.; Yelle, R.; Mendillo, M.; Young, L.; Aylward, A. The thermosphere of Titan simulated by a global three-dimensional time-dependent model. Journal of Geophysical Research: Space Physics 2000, 105, 20833–20856
- Banaszkiewicz \latinet al. 2000 Banaszkiewicz, M.; Lara, L.; Rodrigo, R.; López-Moreno, J.; Molina-Cuberos, G. The upper atmosphere and ionosphere of Titan: A coupled model. Advances in Space Research 2000, 26, 1547–1550
- Szego \latinet al. 2005 Szego, K.; Bebesi, Z.; Erdos, G.; Foldy, L.; Crary, F.; McComas, D.; Young, D.; Bolton, S.; Coates, A.; Rymer, A., \latinet al. The global plasma environment of Titan as observed by Cassini Plasma Spectrometer during the first two close encounters with Titan. Geophysical research letters 2005, 32
- Wahlund \latinet al. 2005 Wahlund, J.-E.; Bostrom, R.; Gustafsson, G.; Gurnett, D.; Kurth, W.; Pedersen, A.; Averkamp, T.; Hospodarsky, G.; Persoon, A.; Canu, P., \latinet al. Cassini measurements of cold plasma in the ionosphere of Titan. Science 2005, 308, 986–989
- Hartle \latinet al. 2006 Hartle, R. E. \latinet al. Initial interpretation of Titan plasma interaction as observed by the Cassini plasma spectrometer: Comparisons with Voyager 1. Plan. & Space Sci. 2006, 54, 1211–1224
- Cravens \latinet al. 2006 Cravens, T. E. \latinet al. Composition of Titan’s ionosphere. Geophysical Research Letters 2006, 33
- Müller-Wodarg \latinet al. 2006 Müller-Wodarg, I.; Yelle, R.; Borggren, N.; Waite Jr, J. Waves and horizontal structures in Titan’s thermosphere. Journal of Geophysical Research: Space Physics 2006, 111
- Coates \latinet al. 2007 Coates, A.; Crary, F.; Lewis, G.; Young, D.; Waite Jr, J.; Sittler Jr, E. Discovery of heavy negative ions in Titan’s ionosphere. Geophysical Research Letters 2007, 34
- Waite \latinet al. 2007 Waite, J. H.; Young, D. T.; Cravens, T. E.; Coates, A. J.; Crary, F. J.; Magee, B.; Westlake, J. The Process of Tholin Formation in Titan’s Upper Atmosphere. Science 2007, 316, 870–
- Müller-Wodarg \latinet al. 2008 Müller-Wodarg, I.; Yelle, R.; Cui, J.; Waite, J. Horizontal structures and dynamics of Titan’s thermosphere. Journal of Geophysical Research: Planets 2008, 113
- Cravens \latinet al. 2008 Cravens, T.; Robertson, I.; Ledvina, S.; Mitchell, D.; Krimigis, S.; Waite Jr, J. Energetic ion precipitation at Titan. Geophysical Research Letters 2008, 35
- Wahlund \latinet al. 2009 Wahlund, J.-E.; Galand, M.; Müller-Wodarg, I.; Cui, J.; Yelle, R.; Crary, F.; Mandt, K.; Magee, B.; Waite Jr, J.; Young, D., \latinet al. On the amount of heavy molecular ions in Titan’s ionosphere. Planetary and Space Science 2009, 57, 1857–1865
- Cui \latinet al. 2009 Cui, J.; Yelle, R. V.; Vuitton, V.; Waite, J. H.; Kasprzak, W. T.; Gell, D. A.; Niemann, H. B.; Müller-Wodarg, I. C. F.; Borggren, N.; Fletcher, G. G.; Patrick, E. L.; Raaen, E.; Magee, B. A. Analysis of Titan’s neutral upper atmosphere from Cassini Ion Neutral Mass Spectrometer measurements. Icarus 2009, 200, 581–615
- Cui \latinet al. 2009 Cui, J.; Galand, M.; Yelle, R. V.; Vuitton, V.; Wahlund, J.-E.; Lavvas, P. P.; Müller-Wodarg, I. C. F.; Cravens, T. E.; Kasprzak, W. T.; Waite, J. H. Diurnal variations of Titan’s ionosphere. Journal of Geophysical Research: Space Physics 2009, 114, n/a–n/a
- Rymer \latinet al. 2009 Rymer, A.; Smith, H.; Wellbrock, A.; Coates, A.; Young, D. Discrete classification and electron energy spectra of Titan’s varied magnetospheric environment. Geophysical Research Letters 2009, 36
- Crary \latinet al. 2009 Crary, F.; Magee, B.; Mandt, K.; Waite Jr, J.; Westlake, J.; Young, D. Heavy ions, temperatures and winds in Titan’s ionosphere: Combined Cassini CAPS and INMS observations. Planetary and Space Science 2009, 57, 1847–1856
- Coates \latinet al. 2009 Coates, A. J.; Wellbrock, A.; Lewis, G. R.; Jones, G. H.; Young, D.; Crary, F.; Waite Jr, J. Heavy negative ions in Titan’s ionosphere: Altitude and latitude dependence. Planetary and Space Science 2009, 57, 1866–1871
- Coates \latinet al. 2012 Coates, A. J.; Wellbrock, A.; Lewis, G. R.; Arridge, C. S.; Crary, F.; Young, D.; Thomsen, M.; Reisenfeld, D.; Sittler Jr, E.; Johnson, R., \latinet al. Cassini in Titan’s tail: CAPS observations of plasma escape. Journal of Geophysical Research: Space Physics 2012, 117
- Ågren \latinet al. 2012 Ågren, K.; Edberg, N.; Wahlund, J.-E. Detection of negative ions in the deep ionosphere of Titan during the Cassini T70 flyby. Geophysical research letters 2012, 39
- Westlake \latinet al. 2011 Westlake, J. H.; Bell, J. M.; Waite, J. H., Jr.; Johnson, R. E.; Luhmann, J. G.; Mandt, K. E.; Magee, B. A.; Rymer, A. M. Titan’s thermospheric response to various plasma environments. Journal of Geophysical Research (Space Physics) 2011, 116, A03318
- Westlake \latinet al. 2012 Westlake, J.; Waite Jr, J.; Mandt, K.; Carrasco, N.; Bell, J.; Magee, B.; Wahlund, J.-E. Titan’s ionospheric composition and structure: Photochemical modeling of Cassini INMS data. Journal of Geophysical Research: Planets 2012, 117
- Snowden \latinet al. 2013 Snowden, D.; Yelle, R.; Cui, J.; Wahlund, J.-E.; Edberg, N.; Ågren, K. The thermal structure of Titan’s upper atmosphere, I: Temperature profiles from Cassini INMS observations. Icarus 2013, 226, 552–582
- Shebanits \latinet al. 2013 Shebanits, O.; Wahlund, J.-E.; Mandt, K.; Ågren, K.; Edberg, N. J.; Waite Jr, J. Negative ion densities in the ionosphere of Titan–Cassini RPWS/LP results. Planetary and Space Science 2013, 84, 153–162
- Teolis \latinet al. 2015 Teolis, B.; Niemann, H.; Waite, J.; Gell, D.; Perryman, R.; Kasprzak, W.; Mandt, K.; Yelle, R.; Lee, A.; Pelletier, F., \latinet al. A revised sensitivity model for Cassini INMS: Results at Titan. Space Science Reviews 2015, 190, 47–84
- Cui \latinet al. 2016 Cui, J.; Cao, Y.-T.; Lavvas, P.; Koskinen, T. T. The variability of HCN in Titan’s upper atmosphere as implied by the Cassini Ion-Neutral Mass Spectrometer measurements. The Astrophysical Journal Letters 2016, 826, L5
- Chatain \latinet al. 2021 Chatain, A.; Wahlund, J.-E.; Shebanits, O.; Hadid, L. Z.; Morooka, M.; Edberg, N. J.; Guaitella, O.; Carrasco, N. Re-Analysis of the Cassini RPWS/LP Data in Titan’s Ionosphere: 1. Detection of Several Electron Populations. Journal of Geophysical Research: Space Physics 2021, 126, e2020JA028412
- Lavvas \latinet al. 2008 Lavvas, P.; Coustenis, A.; Vardavas, I. Coupling photochemistry with haze formation in Titan’s atmosphere, Part I: Model description. Planetary and Space Science 2008, 56, 27 – 66, Surfaces and Atmospheres of the Outer Planets, their Satellites and Ring Systems: Part III, European Geosciences Union General Assembly - Sessions PS3.02 and PS3.03
- Lavvas \latinet al. 2008 Lavvas, P. P.; Coustenis, A.; Vardavas, I. M. Coupling photochemistry with haze formation in Titan’s atmosphere, Part II: Results and validation with Cassini/Huygens data. Plan. & Space Sci. 2008, 56, 67 – 99, Surfaces and Atmospheres of the Outer Planets, their Satellites and Ring Systems: Part III, European Geosciences Union General Assembly - Sessions PS3.02 and PS3.03
- Hörst \latinet al. 2008 Hörst, S. M.; Vuitton, V.; Yelle, R. V. Origin of oxygen species in Titan’s atmosphere. Journal of Geophysical Research (Planets) 2008, 113, E10006
- Krasnopolsky 2009 Krasnopolsky, V. A. A photochemical model of Titan’s atmosphere and ionosphere. Icarus 2009, 201, 226–256
- Krasnopolsky 2010 Krasnopolsky, V. A. The photochemical model of Titan’s atmosphere and ionosphere: A version without hydrodynamic escape. Plan. & Space Sci. 2010, 58, 1507–1515
- Krasnopolsky 2012 Krasnopolsky, V. A. Titan’s photochemical model: Further update, oxygen species, and comparison with Triton and Pluto. Planetary and Space Science 2012, 73, 318–326
- Krasnopolsky 2014 Krasnopolsky, V. A. Chemical composition of Titan’s atmosphere and ionosphere: Observations and the photochemical model. Icarus 2014, 236, 83–91
- Hébrard \latinet al. 2005 Hébrard, E.; Bénilan, Y.; Raulin, F. Sensitivity effects of photochemical parameters uncertainties on hydrocarbon production in the atmosphere of Titan. Advances in Space Research 2005, 36, 268–273, Space Life Sciences: Astrobiology: Steps toward Origin of Life and Titan before Cassini
- Hébrard \latinet al. 2007 Hébrard, E.; Dobrijevic, M.; Bénilan, Y.; Raulin, F. Photochemical kinetics uncertainties in modeling Titan’s atmosphere: First consequences. Planetary and Space Science 2007, 55, 1470–1489
- Hébrard \latinet al. 2009 Hébrard, E.; Dobrijevic, M.; Pernot, P.; Carrasco, N.; Bergeat, A.; Hickson, K. M.; Canosa, A.; Picard, S. D. L.; Sims, I. R. How Measurements of Rate Coefficients at Low Temperature Increase the Predictivity of Photochemical Models of Titan’s Atmosphere. The Journal of Physical Chemistry A 2009, 113, 11227–11237
- Robertson \latinet al. 2009 Robertson, I.; Cravens, T.; Waite Jr, J.; Yelle, R.; Vuitton, V.; Coates, A.; Wahlund, J. E.; Ågren, K.; Mandt, K.; Magee, B., \latinet al. Structure of Titan’s ionosphere: Model comparisons with Cassini data. Planetary and Space Science 2009, 57, 1834–1846
- Hébrard \latinet al. 2012 Hébrard, E.; Dobrijevic, M.; Loison, J. C.; Bergeat, A.; Hickson, K. M. Neutral production of hydrogen isocyanide (HNC) and hydrogen cyanide (HCN) in Titan’s upper atmosphere. Astronomy & Astrophysics 2012, 541, A21
- Hébrard \latinet al. 2013 Hébrard, E.; Dobrijevic, M.; Loison, J. C.; Bergeat, A.; Hickson, K. M.; Caralp, F. Photochemistry of C3Hp hydrocarbons in Titan’s stratosphere revisited. Astronomy & Astrophysics 2013, 552, A132
- Vuitton \latinet al. 2008 Vuitton, V.; Yelle, R. V.; Cui, J. Formation and distribution of benzene on Titan. Journal of Geophysical Research 2008, 113
- Vuitton \latinet al. 2009 Vuitton, V.; Yelle, R. V.; Lavvas, P. Composition and chemistry of Titan’s thermosphere and ionosphere. Royal Society of London Philosophical Transactions Series A 2009, 367, 729–741
- Vuitton \latinet al. 2012 Vuitton, V.; Yelle, R. V.; Lavvas, P.; Klippenstein, S. J. Rapid Association Reactions at Low Pressure: Impact on the Formation of Hydrocarbons on Titan. Astrophys. J. 2012, 744, 11
- Bell \latinet al. 2010 Bell, J. M.; Bougher, S. W.; Waite, J. H.; Ridley, A. J.; Magee, B. A.; Mandt, K. E.; Westlake, J.; DeJong, A. D.; Bar-Nun, A.; Jacovi, R.; Toth, G.; De La Haye, V. Simulating the one-dimensional structure of Titan’s upper atmosphere: 1. Formulation of the Titan Global Ionosphere-Thermosphere Model and benchmark simulations. Journal of Geophysical Research (Planets) 2010, 115, E12002
- Lara \latinet al. 2014 Lara, L. M.; Lellouch, E.; González, M.; Moreno, R.; Rengel, M. A time-dependent photochemical model for Titan’s atmosphere and the origin of H2O. Astronomy & Astrophysics 2014, 566, A143
- Dobrijevic \latinet al. 2014 Dobrijevic, M.; Hébrard, E.; Loison, J.; Hickson, K. Coupling of oxygen, nitrogen, and hydrocarbon species in the photochemistry of Titan’s atmosphere. Icarus 2014, 228, 324–346
- Loison \latinet al. 2015 Loison, J.; Hébrard, E.; Dobrijevic, M.; Hickson, K.; Caralp, F.; Hue, V.; Gronoff, G.; Venot, O.; Bénilan, Y. The neutral photochemistry of nitriles, amines and imines in the atmosphere of Titan. Icarus 2015, 247, 218–247
- Li \latinet al. 2015 Li, C.; Zhang, X.; Gao, P.; Yung, Y. Vertical Distribution of C3 Hydrocarbons in the Stratosphere of Titan. The Astrophysical Journal 2015, 803, L19
- Willacy \latinet al. 2016 Willacy, K.; Allen, M.; Yung, Y. A New Astrobiological Model of the Atmosphere of Titan. The Astrophysical Journal 2016, 829, 79
- Dobrijevic \latinet al. 2016 Dobrijevic, M.; Loison, J.; Hickson, K.; Gronoff, G. 1D-coupled photochemical model of neutrals, cations and anions in the atmosphere of Titan. Icarus 2016, 268, 313–339
- Loison \latinet al. 2019 Loison, J.; Dobrijevic, M.; Hickson, K. The photochemical production of aromatics in the atmosphere of Titan. Icarus 2019, 329, 55–71
- Vuitton \latinet al. 2019 Vuitton, V.; Yelle, R. V.; Klippenstein, S. J.; Hörst, S. M.; Lavvas, P. Simulating the density of organic species in the atmosphere of Titan with a coupled ion-neutral photochemical model. Icarus 2019,
- Khare \latinet al. 2002 Khare, B. N.; Bakes, E.; Imanaka, H.; McKay, C. P.; Cruikshank, D. P.; Arakawa, E. T. Analysis of the time-dependent chemical evolution of Titan haze tholin. Icarus 2002, 160, 172–182
- Sagan \latinet al. 1992 Sagan, C.; Thompson, W. R.; Khare, B. N. Titan: a laboratory for prebiological organic chemistry. Accounts of Chemical Research 1992, 25, 286–292
- Wilson and Atreya 2003 Wilson, E.; Atreya, S. Chemical sources of haze formation in Titan’s atmosphere. Planetary and Space Science 2003, 51, 1017–1033
- Trainer \latinet al. 2004 Trainer, M. G.; Pavlov, A. A.; Jimenez, J. L.; McKay, C. P.; Worsnop, D. R.; Toon, O. B.; Tolbert, M. A. Chemical composition of Titan’s haze: Are PAHs present? Geophysical research letters 2004, 31
- Imanaka \latinet al. 2004 Imanaka, H.; Khare, B. N.; Elsila, J. E.; Bakes, E. L.; McKay, C. P.; Cruikshank, D. P.; Sugita, S.; Matsui, T.; Zare, R. N. Laboratory experiments of Titan tholin formed in cold plasma at various pressures: implications for nitrogen-containing polycyclic aromatic compounds in Titan haze. Icarus 2004, 168, 344–366
- Sekine \latinet al. 2008 Sekine, Y.; Imanaka, H.; Matsui, T.; Khare, B. N.; Bakes, E. L.; McKay, C. P.; Sugita, S. The role of organic haze in Titan’s atmospheric chemistry: I. Laboratory investigation on heterogeneous reaction of atomic hydrogen with Titan tholin. Icarus 2008, 194, 186–200
- Imanaka \latinet al. 2012 Imanaka, H.; Cruikshank, D. P.; Khare, B. N.; McKay, C. P. Optical constants of Titan tholins at mid-infrared wavelengths (2.5–25 m) and the possible chemical nature of Titan’s haze particles. Icarus 2012, 218, 247–261
- Cable \latinet al. 2011 Cable, M. L.; Hörst, S. M.; Hodyss, R.; Beauchamp, P. M.; Smith, M. A.; Willis, P. A. Titan Tholins: Simulating Titan Organic Chemistry in the Cassini-Huygens Era. Chemical Reviews 2011, 112, 1882–1909
- Hörst \latinet al. 2018 Hörst, S. M.; Yoon, Y. H.; Ugelow, M. S.; Parker, A. H.; Li, R.; de Gouw, J. A.; Tolbert, M. A. Laboratory investigations of Titan haze formation: In situ measurement of gas and particle composition. Icarus 2018, 301, 136–151
- Lavvas \latinet al. 2010 Lavvas, P.; Yelle, R.; Griffith, C. Titan’s vertical aerosol structure at the Huygens landing site: Constraints on particle size, density, charge, and refractive index. Icarus 2010, 210, 832–842
- Lorenz 1993 Lorenz, R. D. The life, death and afterlife of a raindrop on Titan. Planetary and Space Science 1993, 41, 647–655
- Lorenz 1995 Lorenz, R. Raindrops on Titan. Advances in Space Research 1995, 15, 317–320
- McKay \latinet al. 2001 McKay, C.; Coustenis, A.; Samuelson, R.; Lemmon, M.; Lorenz, R.; Cabane, M.; Rannou, P.; Drossart, P. Physical properties of the organic aerosols and clouds on Titan. Planetary and Space Science 2001, 49, 79–99
- Karkoschka and Tomasko 2009 Karkoschka, E.; Tomasko, M. G. Rain and dewdrops on titan based on in situ imaging. Icarus 2009, 199, 442–448
- Lorenz \latinet al. 2006 Lorenz, R. D. \latinet al. The Sand Seas of Titan: Cassini RADAR Observations of Longitudinal Dunes. Science 2006, 312, 724–727
- Radebaugh \latinet al. 2008 Radebaugh, J.; Lorenz, R.; Lunine, J.; Wall, S.; Boubin, G.; Reffet, E.; Kirk, R. L.; Lopes, R.; Stofan, E.; Soderblom, L., \latinet al. Dunes on Titan observed by Cassini RADAR. Icarus 2008, 194, 690–703
- Mastrogiuseppe \latinet al. 2014 Mastrogiuseppe, M.; Poggiali, V.; Seu, R.; Martufi, R.; Notarnicola, C. Titan dune heights retrieval by using Cassini Radar Altimeter. Icarus 2014, 230, 191–197
- Fulchignoni \latinet al. 2005 Fulchignoni, M. \latinet al. In situ measurements of the physical characteristics of Titan’s environment. Nature 2005, 438, 785–791
- M. Fulchignoni \latinet al. 2002 M. Fulchignoni, M. \latinet al. The Characterisation of Titan’s Atmospheric Physical Properties by the Huygens Atmospheric Structure Instrument (HASI). Space Science Reviews 2002, 104, 395–431
- Kliore \latinet al. 2004 Kliore, A. J.; Anderson, J. D.; Armstrong, J. W.; Asmar, S. W.; Hamilton, C. L.; Rappaport, N. J.; Wahlquist, H. D.; Ambrosini, R.; Flasar, F. M.; French, R. G.; Iess, L.; Marouf, E. A.; Nagy, A. F. Cassini Radio Science. Space Sci. Rev. 2004, 115, 1–70
- Schinder \latinet al. 2011 Schinder, P. J.; Flasar, F. M.; Marouf, E. A.; French, R. G.; McGhee, C. A.; Kliore, A. J.; Rappaport, N. J.; Barbinis, E.; Fleischman, D.; Anabtawi, A. The structure of Titan’s atmosphere from Cassini radio occultations. Icarus 2011, 215, 460–474
- Schinder \latinet al. 2012 Schinder, P. J.; Flasar, F. M.; Marouf, E. A.; French, R. G.; McGhee, C. A.; Kliore, A. J.; Rappaport, N. J.; Barbinis, E.; Fleischman, D.; Anabtawi, A. The structure of Titan’s atmosphere from Cassini radio occultations: Occultations from the Prime and Equinox missions. Icarus 2012, 221, 1020–1031
- Schinder \latinet al. 2020 Schinder, P. J.; Flasar, F. M.; Marouf, E. A.; French, R. G.; Anabtawi, A.; Barbinis, E.; Fleischman, D.; Achterberg, R. K. The structure of Titan’s atmosphere from Cassini radio occultations: One- and two-way occultations. Icarus 2020, 345, 113720
- Teanby \latinet al. 2019 Teanby, N. A.; Sylvestre, M.; Sharkey, J.; Nixon, C. A.; Vinatier, S.; Irwin, P. G. J. Seasonal Evolution of Titan’s Stratosphere During the Cassini Mission. Geophysical Research Letters 2019, 46, 3079–3089
- Lellouch \latinet al. 2019 Lellouch, E.; Gurwell, M. A.; Moreno, R.; Vinatier, S.; Strobel, D. F.; Moullet, A.; Butler, B.; Lara, L.; Hidayat, T.; Villard, E. An intense thermospheric jet on Titan. Nature Astronomy 2019, 3, 614–619
- Yelle 1991 Yelle, R. V. Non-LTE models of Titan’s upper atmosphere. The Astrophysical Journal 1991, 383, 380
- Flasar \latinet al. 2004 Flasar, F. M. \latinet al. Exploring The Saturn System In The Thermal Infrared: The Composite Infrared Spectrometer. Space Sci. Rev. 2004, 115, 169–297
- Flasar \latinet al. 2005 Flasar, F. M. \latinet al. Titan’s Atmospheric Temperatures, Winds, and Composition. Science 2005, 308, 975–978
- Achterberg \latinet al. 2008 Achterberg, R. K.; Conrath, B. J.; Gierasch, P. J.; Flasar, F. M.; Nixon, C. A. Titan’s middle-atmospheric temperatures and dynamics observed by the Cassini Composite Infrared Spectrometer. Icarus 2008, 194, 263–277
- Serigano \latinet al. 2016 Serigano, J.; Nixon, C. A.; Cordiner, M. A.; Irwin, P. G. J.; Teanby, N. A.; Charnley, S. B.; Lindberg, J. E. Isotopic Ratios Of Carbon And Oxygen In Titan’s CO Using ALMA. The Astrophysical Journal 2016, 821, L8
- Thelen \latinet al. 2018 Thelen, A. E.; Nixon, C.; Chanover, N.; Molter, E.; Cordiner, M.; Achterberg, R.; Serigano, J.; Irwin, P.; Teanby, N.; Charnley, S. Spatial variations in Titan’s atmospheric temperature: ALMA and Cassini comparisons from 2012 to 2015. Icarus 2018, 307, 380–390
- Strobel \latinet al. 2009 Strobel, D. F.; Atreya, S. K.; Bézard, B.; Ferri, F.; Flasar, F. M.; Fulchignoni, M.; Lellouch, E.; Müller-Wodarg, I. Titan from Cassini-Huygens; Springer Netherlands, 2009; pp 235–257
- Achterberg \latinet al. 2011 Achterberg, R. K.; Gierasch, P. J.; Conrath, B. J.; Michael Flasar, F.; Nixon, C. A. Temporal variations of Titan’s middle-atmospheric temperatures from 2004 to 2009 observed by Cassini/CIRS. Icarus 2011, 211, 686–698
- Teanby \latinet al. 2012 Teanby, N. A.; Irwin, P. G. J.; Nixon, C. A.; de Kok, R.; Vinatier, S.; Coustenis, A.; Sefton-Nash, E.; Calcutt, S. B.; Flasar, F. M. Active upper-atmosphere chemistry and dynamics from polar circulation reversal on Titan. Nature 2012, 491, 732–735
- Lellouch \latinet al. 1990 Lellouch, E.; Hunten, D. M.; Kockarts, G.; Coustenis, A. Titan’s thermosphere profile. Icarus 1990, 83, 308–324
- Gurnett \latinet al. 2004 Gurnett, D. A. \latinet al. The Cassini Radio and Plasma Wave Investigation. Space Science Reviews 2004, 114, 395–463
- Ågren \latinet al. 2009 Ågren, K.; Wahlund, J.-E.; Garnier, P.; Modolo, R.; Cui, J.; Galand, M.; Müller-Wodarg, I. On the ionospheric structure of Titan. Planetary and Space Science 2009, 57, 1821–1827
- Bell \latinet al. 2014 Bell, J. M.; Waite, J. H.; Westlake, J. H.; Bougher, S. W.; Ridley, A. J.; Perryman, R.; Mandt, K. Developing a self-consistent description of Titan’s upper atmosphere without hydrodynamic escape. Journal of Geophysical Research: Space Physics 2014, 119, 4957–4972
- Edberg \latinet al. 2013 Edberg, N. J. T.; Andrews, D. J.; Shebanits, O.; Ågren, K.; Wahlund, J.-E.; Opgenoorth, H. J.; Cravens, T. E.; Girazian, Z. Solar cycle modulation of Titan’s ionosphere. Journal of Geophysical Research: Space Physics 2013, 118, 5255–5264
- Garnier \latinet al. 2009 Garnier, P.; Wahlund, J.-E.; Rosenqvist, L.; Modolo, R.; Ågren, K.; Sergis, N.; Canu, P.; Andre, M.; Gurnett, D. A.; Kurth, W. S.; Krimigis, S. M.; Coates, A.; Dougherty, M.; Waite, J. H. Titan's ionosphere in the magnetosheath: Cassini RPWS results during the T32 flyby. Annales Geophysicae 2009, 27, 4257–4272
- Snowden \latinet al. 2011 Snowden, D.; Winglee, R.; Kidder, A. Titan at the edge: 1. Titan’s interaction with Saturn’s magnetosphere in the prenoon sector. Journal of Geophysical Research: Space Physics 2011, 116
- Snowden \latinet al. 2011 Snowden, D.; Winglee, R.; Kidder, A. Titan at the edge: 2. A global simulation of Titan exiting and reentering Saturn’s magnetosphere at 13:16 Saturn local time. Journal of Geophysical Research: Space Physics 2011, 116
- Edberg \latinet al. 2013 Edberg, N. J. T.; Andrews, D. J.; Shebanits, O.; Ågren, K.; Wahlund, J.-E.; Opgenoorth, H. J.; Roussos, E.; Garnier, P.; Cravens, T. E.; Badman, S. V.; Modolo, R.; Bertucci, C.; Dougherty, M. K. Extreme densities in Titan’s ionosphere during the T85 magnetosheath encounter. Geophysical Research Letters 2013, 40, 2879–2883
- Thelen \latinet al. 2022 Thelen, A. E.; Nixon, C. A.; Cosentino, R. G.; Cordiner, M. A.; Teanby, N. A.; Newman, C. E.; Irwin, P. G.; Charnley, S. B. Variability in Titan’s Mesospheric HCN and Temperature Structure as Observed by ALMA. The Planetary Science Journal 2022, 3, 146
- Cui \latinet al. 2008 Cui, J.; Yelle, R. V.; Volk, K. Distribution and escape of molecular hydrogen in Titan's thermosphere and exosphere. Journal of Geophysical Research 2008, 113
- Schunk and Nagy 2004 Schunk, R. W.; Nagy, A. F. In Ionospheres: physics, plasma physics, and chemistry; Dressler, A., Houghton, J., Rycroft, M., Eds.; Cambridge atmospheric and space science series; Cambridge University Press, 2004; pp 104–147
- Hourdin \latinet al. 1995 Hourdin, F.; Talagrand, O.; Sadourny, R.; Courtin, R.; Gautier, D.; Mckay, C. P. Numerical simulation of the general circulation of the atmosphere of Titan. Icarus 1995, 117, 358–374
- Lebonnois \latinet al. 2001 Lebonnois, S.; Toublanc, D.; Hourdin, F.; Rannou, P. Seasonal variations of Titan’s atmospheric composition. Icarus 2001, 152, 384–406
- Hourdin \latinet al. 2004 Hourdin, F.; Lebonnois, S.; Luz, D.; Rannou, P. Titan’s stratospheric composition driven by condensation and dynamics. Journal of Geophysical Research: Planets 2004, 109
- Rannou \latinet al. 2004 Rannou, P.; Hourdin, F.; Mckay, C. P.; Luz, D. A coupled dynamics-microphysics model of Titan’s atmosphere. Icarus 2004, 170, 443–462
- Lebonnois \latinet al. 2009 Lebonnois, S.; Rannou, P.; Hourdin, F. The coupling of winds, aerosols and chemistry in Titan’s atmosphere. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 2009, 367, 665–682
- Lora \latinet al. 2019 Lora, J. M.; Tokano, T.; Vatant d’Ollone, J.; Lebonnois, S.; Lorenz, R. D. A model intercomparison of Titan’s climate and low-latitude environment. Icarus 2019, 333, 113–126
- Vinatier \latinet al. 2020 Vinatier, S.; Mathé, C.; Bézard, B.; d’Ollone, J. V.; Lebonnois, S.; Dauphin, C.; Flasar, F.; Achterberg, R.; Seignovert, B.; Sylvestre, M., \latinet al. Temperature and chemical species distributions in the middle atmosphere observed during Titan’s late northern spring to early summer. Astronomy & Astrophysics 2020, 641, A116
- Mathé \latinet al. 2020 Mathé, C.; Vinatier, S.; Bézard, B.; Lebonnois, S.; Gorius, N.; Jennings, D. E.; Mamoutkine, A.; Guandique, E.; D’ollone, J. V. Seasonal changes in the middle atmosphere of Titan from Cassini/CIRS observations: Temperature and trace species abundance profiles from 2004 to 2017. Icarus 2020, 344, 113547
- Sylvestre \latinet al. 2020 Sylvestre, M.; Teanby, N. A.; d’Ollone, J. V.; Vinatier, S.; Bézard, B.; Lebonnois, S.; Irwin, P. G. Seasonal evolution of temperatures in Titan’s lower stratosphere. Icarus 2020, 344, 113188
- Cordiner \latinet al. 2020 Cordiner, M. A.; Garcia-Berrios, E.; Cosentino, R. G.; Teanby, N. A.; Newman, C. E.; Nixon, C. A.; Thelen, A. E.; Charnley, S. B. Detection of Dynamical Instability in Titan’s Thermospheric Jet. The Astrophysical Journal 2020, 904, L12
- Coustenis \latinet al. 2020 Coustenis, A.; Jennings, D.; Achterberg, R.; Lavvas, P.; Bampasidis, G.; Nixon, C.; Flasar, F. M. Titan’s neutral atmosphere seasonal variations up to the end of the Cassini mission. Icarus 2020, 344, 113413
- Sharkey \latinet al. 2021 Sharkey, J.; Teanby, N. A.; Sylvestre, M.; Mitchell, D. M.; Seviour, W. J.; Nixon, C. A.; Irwin, P. G. Potential vorticity structure of Titan’s polar vortices from Cassini CIRS observations. Icarus 2021, 354, 114030
- Teanby \latinet al. 2007 Teanby, N. A.; Irwin, P. G. J.; de Kok, R.; Vinatier, S.; Bézard, B.; Nixon, C. A.; Flasar, F. M.; Calcutt, S. B.; Bowles, N. E.; Fletcher, L.; Howett, C.; Taylor, F. W. Vertical profiles of HCN, HC3N, and C2H2 in Titan’s atmosphere derived from Cassini/CIRS data. Icarus 2007, 186, 364–384
- Vinatier \latinet al. 2007 Vinatier, S.; Bézard, B.; Fouchet, T.; Teanby, N. A.; de Kok, R.; Irwin, P. G. J.; Conrath, B. J.; Nixon, C. A.; Romani, P. N.; Flasar, F. M.; Coustenis, A. Vertical abundance profiles of hydrocarbons in Titan’s atmosphere at 15∘ S and 80∘ N retrieved from Cassini/CIRS spectra. Icarus 2007, 188, 120–138
- Marten \latinet al. 2002 Marten, A.; Hidayat, T.; Biraud, Y.; Moreno, R. New Millimeter Heterodyne Observations of Titan: Vertical Distributions of Nitriles HCN, HC3N, CH3CN, and the Isotopic Ratio 15N/14N in Its Atmosphere. Icarus 2002, 158, 532–544
- Nixon \latinet al. 2020 Nixon, C. A.; Thelen, A. E.; Cordiner, M. A.; Kisiel, Z.; Charnley, S. B.; Molter, E. M.; Serigano, J.; Irwin, P. G. J.; Teanby, N. A.; Kuan, Y.-J. Detection of Cyclopropenylidene on Titan with ALMA. The Astronomical Journal 2020, 160, 205
- Palmer \latinet al. 2017 Palmer, M. Y.; Cordiner, M. A.; Nixon, C. A.; Charnley, S. B.; Teanby, N. A.; Kisiel, Z.; Irwin, P. G. J.; Mumma, M. J. ALMA Detection and Astrobiological Potential of Vinyl Cyanide on Titan. Science Advances 2017, 3, e1700022
- Cordiner \latinet al. 2015 Cordiner, M.; Palmer, M.; Nixon, C.; Irwin, P.; Teanby, N.; Charnley, S.; Mumma, M.; Kisiel, Z.; Serigano, J.; Kuan, Y.-J., \latinet al. Ethyl cyanide on Titan: Spectroscopic detection and mapping using Alma. The Astrophysical Journal Letters 2015, 800, L14
- Thelen \latinet al. 2020 Thelen, A. E.; Cordiner, M. A.; Nixon, C. A.; Vuitton, V.; Kisiel, Z.; Charnley, S. B.; Palmer, M. Y.; Teanby, N. A.; Irwin, P. G. J. Detection of CH3C3N in Titan’s Atmosphere. The Astrophysical Journal 2020, 903, L22
- Thelen \latinet al. 2019 Thelen, A. E.; Nixon, C.; Chanover, N.; Cordiner, M.; Molter, E.; Teanby, N.; Irwin, P.; Serigano, J.; Charnley, S. Abundance measurements of Titan’s stratospheric HCN, HC3N, C3H4, and CH3CN from ALMA observations. Icarus 2019, 319, 417–432
- Lombardo \latinet al. 2019 Lombardo, N. A.; Nixon, C. A.; Sylvestre, M.; Jennings, D. E.; Teanby, N.; Irwin, P. J. G.; Flasar, F. M. Ethane in Titan’s Stratosphere from Cassini CIRS Far- and Mid-infrared Spectra. The Astronomical Journal 2019, 157, 160
- Lombardo \latinet al. 2019 Lombardo, N. A.; Nixon, C. A.; Greathouse, T. K.; Bézard, B.; Jolly, A.; Vinatier, S.; Teanby, N. A.; Richter, M. J.; G Irwin, P. J.; Coustenis, A.; et al., Detection of Propadiene on Titan. The Astrophysical Journal 2019, 881, L33
- Cravens \latinet al. 2005 Cravens, T. E. \latinet al. Titan’s ionosphere: Model comparisons with Cassini Ta data. Geophysical Research Letters 2005, 32, n/a–n/a
- Lavvas \latinet al. 2011 Lavvas, P.; Galand, M.; Yelle, R.; Heays, A.; Lewis, B.; Lewis, G.; Coates, A. Energy deposition and primary chemical products in Titan’s upper atmosphere. Icarus 2011, 213, 233–251
- Mount \latinet al. 1977 Mount, G. H.; Warden, E.; Moos, H. Photoabsorption cross sections of methane from 1400 to 1850 A. Astrophysical Journal, Part 2-Letters to the Editor, vol. 214, May 15, 1977, p. L47-L49. 1977, 214, L47–L49
- Erwin and Kunc 2005 Erwin, D. A.; Kunc, J. A. Electron-impact dissociation of the methane molecule into neutral fragments. Physical Review A 2005, 72, 052719
- Lee and Chiang 1983 Lee, L.; Chiang, C. Fluorescence yield from photodissociation of CH4 at 1060–1420 Å. The Journal of Chemical Physics 1983, 78, 688–691
- Capone \latinet al. 1976 Capone, L.; Whitten, R.; Dubach, J.; Prasad, S.; Huntress, W. The lower ionosphere of Titan. Icarus 1976, 28, 367–378
- Capone \latinet al. 1983 Capone, L. A.; Dubach, J.; Prasad, S. S.; Whitten, R. C. Galactic cosmic rays and N2 dissociation on Titan. Icarus 1983, 55, 73–82
- Molina-Cuberos \latinet al. 1999 Molina-Cuberos, G.; López-Moreno, J.; Rodrigo, R.; Lara, L.; O’Brien, K. Ionization by cosmic rays of the atmosphere of Titan. Planetary and Space Science 1999, 47, 1347–1354
- Molina-Cuberos \latinet al. 1999 Molina-Cuberos, G. J.; López-Moreno, J. J.; Rodrigo, R.; Lara, L. M. Chemistry of the galactic cosmic ray induced ionosphere of Titan. Journal of Geophysical Research: Planets 1999, 104, 21997–22024
- Aoto \latinet al. 2006 Aoto, T.; Ito, K.; Hikosaka, Y.; Shibasaki, A.; Hirayama, R.; Yamamono, N.; Miyoshi, E. Inner-valence states of N and the dissociation dynamics studied by threshold photoelectron spectroscopy and configuration interaction calculation. The Journal of chemical physics 2006, 124, 234306
- Shaw \latinet al. 1992 Shaw, D.; Holland, D.; MacDonald, M.; Hopkirk, A.; Hayes, M.; McSweeney, S. A study of the absolute photoabsorption cross section and the photionization quantum efficiency of nitrogen from the ionization threshold to 485 Å. Chemical physics 1992, 166, 379–391
- Mitsuke \latinet al. 1991 Mitsuke, K.; Suzuki, S.; Imamura, T.; Koyano, I. Negative-ion mass spectrometric study of ion-pair formation in the vacuum ultraviolet. IV. CH H- + CH and CD D CD. The Journal of Chemical Physics 1991, 94, 6003–6006
- Ruscic and Berkowitz 1990 Ruscic, B.; Berkowitz, J. Photoion-pair formation and photoelectron-induced dissociative attachment in C2H2: (HCC–H). The Journal of Chemical Physics 1990, 93, 5586–5593
- Rawat \latinet al. 2008 Rawat, P.; Prabhudesai, V. S.; Rahman, M.; Ram, N. B.; Krishnakumar, E. Absolute cross sections for dissociative electron attachment to NH3 and CH4. International Journal of Mass Spectrometry 2008, 277, 96–102
- Stamatovic and Schulz 1970 Stamatovic, A.; Schulz, G. Dissociative Attachment in CO and Formation of C-. The Journal of Chemical Physics 1970, 53, 2663–2667
- Herbst and Osamura 2008 Herbst, E.; Osamura, Y. Calculations on the formation rates and mechanisms for CnH anions in interstellar and circumstellar media. The Astrophysical Journal 2008, 679, 1670
- Kamińska \latinet al. 2010 Kamińska, M.; Zhaunerchyk, V.; Vigren, E.; Danielsson, M.; Hamberg, M.; Geppert, W. D.; Larsson, M.; Rosén, S.; Thomas, R. D.; Semaniak, J. Dissociative recombination of CH and CD: Measurement of the product branching fractions and the absolute cross sections, and the breakup dynamics in the CH3 + H + H product channel. Physical Review A 2010, 81, 062701
- Semaniak \latinet al. 1998 Semaniak, J.; Larson, Å.; Le Padellec, A.; Strömholm, C.; Larsson, M.; Rosen, S.; Peverall, R.; Danared, H.; Djuric, N.; Dunn, G., \latinet al. Dissociative recombination and excitation of CH: Absolute cross sections and branching fractions. The Astrophysical Journal 1998, 498, 886
- Klippenstein \latinet al. 1996 Klippenstein, S. J.; Yang, Y.-C.; Ryzhov, V.; Dunbar, R. C. Theory and modeling of ion–molecule radiative association kinetics. The Journal of chemical physics 1996, 104, 4502–4516
- Gerlich and Horning 1992 Gerlich, D.; Horning, S. Experimental investigation of radiative association processes as related to interstellar chemistry. Chemical Reviews 1992, 92, 1509–1539
- Luca \latinet al. 2002 Luca, A.; Voulot, D.; Gerlich, D. Low temperature reactions between stored ions and condensable gases: formation of protonated methanol via radiative association. WDS. 2002; pp 294–300
- Herbst 1980 Herbst, E. A New Look At Radiative Association In Dense Interstellar Clouds. The Astrophysical Journal 1980, 237, 462–470
- Herbst 1985 Herbst, E. An update of and suggested increase in calculated radiative association rate coefficients. The Astrophysical journal; An update of and suggested increase in calculated radiative association rate coefficients 1985, 291, 226–229
- Herbst and Dunbar 1991 Herbst, E.; Dunbar, R. C. A global view of radiative association as a function of product size : interstellar implications. Monthly Notices of the Royal Astronomical Society; A global view of radiative association as a function of product size : interstellar implications 1991, 253, 341–349
- Herbst 2021 Herbst, E. Unusual Chemical Processes in Interstellar Chemistry: Past and Present. Frontiers in Astronomy and Space Sciences 2021, 8
- Kaiser 2002 Kaiser, R. I. Experimental investigation on the formation of carbon-bearing molecules in the interstellar medium via neutral- neutral reactions. Chemical Reviews 2002, 102, 1309–1358
- Lindinger \latinet al. 2000 Lindinger, W.; Hansel, A.; Herman, Z. Ion—Molecule Reactions. Advances in Atomic Molecular and Optical Physics 2000, 43, 243–294
- Opansky and Leone 1996 Opansky, B. J.; Leone, S. R. Low-Temperature Rate Co-efficients of C2H with CH4 and CD4 from 154 to 359 K. J. Phys. Chem. 1996, 100, 4888–4892
- Vuitton \latinet al. 2006 Vuitton, V.; Doussin, J.-F.; Bénilan, Y.; Raulin, F.; Gazeau, M.-C. Experimental and theoretical study of hydrocarbon photochemistry applied to Titan stratosphere. Icarus 2006, 185, 287–300
- Herbert \latinet al. 1992 Herbert, L.; Smith, I. W.; Spencer-smith, R. D. Rate constants for the elementary reactions between CN radicals and CH4, C2H6, C2H4, C3H6, and C2H2 in the range: 295 T/K 700. International journal of chemical kinetics 1992, 24, 791–802
- Sims \latinet al. 1993 Sims, I. R.; Queffelec, J.-L.; Travers, D.; Rowe, B. R.; Herbert, L. B.; Karthäuser, J.; Smith, I. W. Rate constants for the reactions of CN with hydrocarbons at low and ultra-low temperatures. Chemical Physics Letters 1993, 211, 461–468
- Gannon \latinet al. 2007 Gannon, K. L.; Glowacki, D. R.; Blitz, M. A.; Hughes, K. J.; Pilling, M. J.; Seakins, P. W. H Atom Yields from the Reactions of CN Radicals with C2H2, C2H4, C3H6, C4H8, and C4H8. The Journal of Physical Chemistry A 2007, 111, 6679–6692
- McKee \latinet al. 2003 McKee, K.; Blitz, M. A.; Hughes, K. J.; Pilling, M. J.; Qian, H.-B.; Taylor, A.; Seakins, P. W. H Atom Branching Ratios from the Reactions of CH with C2H2, C2H4, C2H6, and neo-C5H12 at Room Temperature and 25 Torr. The Journal of Physical Chemistry A 2003, 107, 5710–5716
- Berman \latinet al. 1982 Berman, M. R.; Fleming, J.; Harvey, A.; Lin, M.-C. Temperature dependence of the reactions of CH radicals with unsaturated hydrocarbons. Chemical physics 1982, 73, 27–33
- Thiesemann \latinet al. 2001 Thiesemann, H.; Clifford, E. P.; Taatjes, C. A.; Klippenstein, S. J. Temperature dependence and deuterium kinetic isotope effects in the CH (CD)+ C2H4 (C2D4) reaction between 295 and 726 K. The Journal of Physical Chemistry A 2001, 105, 5393–5401
- Slagle \latinet al. 1988 Slagle, I. R.; Gutman, D.; Davies, J. W.; Pilling, M. J. Study of the recombination reaction CH3+CH C2H6. I: Experiment. Journal of physical chemistry (1952); Study of the recombination reaction CH3+CH3→C2H6. I: Experiment 1988, 92, 2455–2462
- Brouard \latinet al. 1989 Brouard, M.; Macpherson, M. T.; Pilling, M. J. Experimental and RRKM modeling study of the methyl hydrogen atom and deuterium atom reactions. The Journal of Physical Chemistry 1989, 93, 4047–4059
- Alnama \latinet al. 2007 Alnama, K.; Boyé-Péronne, S.; Douin, S.; Innocenti, F.; O’Reilly, J.; Roche, A.-L.; Shafizadeh, N.; Zuin, L.; Gauyacq, D. Photolysis of allene and propyne in the 7–30 eV region probed by the visible fluorescence of their fragments. The Journal of chemical physics 2007, 126, 044304
- Herbst \latinet al. 2000 Herbst, E.; Terzieva, R.; Talbi, D. Calculations on the rates, mechanisms, and interstellar importance of the reactions between C and NH2 and between N and CH2. Monthly Notices of The Royal Astronomical Society 2000, 311, 869–876
- Courtin \latinet al. 1991 Courtin, R.; Wagener, R.; McKay, C. P.; Caldwell, J.; Fricke, K.-H.; Raulin, F.; Bruston, P. UV spectroscopy of Titan’s atmosphere, planetary organic chemistry and prebiological synthesis: II. Interpretation of new IUE observations in the 220–335 nm Range. Icarus 1991, 90, 43–56
- Cabane \latinet al. 1993 Cabane, M.; Rannou, P.; Chassefière, E.; Israel, G. Fractal aggregates in Titan’s atmosphere. Planetary and Space Science 1993, 41, 257–267
- Clarke and Ferris 1997 Clarke, D. W.; Ferris, J. P. Titan haze: structure and properties of cyanoacetylene and cyanoacetylene–acetylene photopolymers. Icarus 1997, 127, 158–172
- Lara \latinet al. 1999 Lara, L.-M.; Lellouch, E.; Shematovich, V. Titan’s atmospheric haze: the case for HCN incorporation. Astronomy and Astrophysics 1999, 341, 312–317
- Lebonnois \latinet al. 2002 Lebonnois, S.; Bakes, E.; McKay, C. P. Transition from gaseous compounds to aerosols in Titan’s atmosphere. Icarus 2002, 159, 505–517
- Tran \latinet al. 2003 Tran, B. N.; Ferris, J. P.; Chera, J. J. The photochemical formation of a Titan haze analog. Structural analysis by X-ray photoelectron and infrared spectroscopy. Icarus 2003, 162, 114–124
- Tran \latinet al. 2003 Tran, B. N.; Joseph, J. C.; Ferris, J. P.; Persans, P. D.; Chera, J. J. Simulation of Titan haze formation using a photochemical flow reactor: The optical constants of the polymer. Icarus 2003, 165, 379–390
- Perrin \latinet al. 2021 Perrin, Z.; Carrasco, N.; Chatain, A.; Jovanovic, L.; Vettier, L.; Ruscassier, N.; Cernogora, G. An atmospheric origin for HCN-derived polymers on Titan. Processes 2021, 9, 965
- Vuitton \latinet al. 2007 Vuitton, V.; Yelle, R. V.; McEwan, M. J. Ion chemistry and N-containing molecules in Titan’s upper atmosphere. Icarus 2007, 191, 722–742
- Magee \latinet al. 2009 Magee, B. A.; Waite, J. H.; Mandt, K. E.; Westlake, J.; Bell, J.; Gell, D. A. INMS-derived composition of Titan’s upper atmosphere: Analysis methods and model comparison. Plan. & Space Sci. 2009, 57, 1895–1916
- Walter \latinet al. 1991 Walter, D.; Grotheer, H.-H.; Davies, J. W.; Pilling, M. J.; Wagner, A. F. Experimental and theoretical study of the recombination reaction CH3 + CH3 C2H6. Symposium (International) on Combustion. 1991; pp 107–114
- Cody \latinet al. 2003 Cody, R. J.; Romani, P. N.; Nesbitt, F. L.; Iannone, M. A.; Tardy, D. C.; Stief, L. J. Rate constant for the reaction CH3+ CH3 C2H6 at T= 155 K and model calculation of the CH3 abundance in the atmospheres of Saturn and Neptune. Journal of Geophysical Research: Planets 2003, 108
- Baulch \latinet al. 2005 Baulch, D.; Bowman, C.; Cobos, C. J.; Cox, R. A.; Just, T.; Kerr, J.; Pilling, M.; Stocker, D.; Troe, J.; Tsang, W., \latinet al. Evaluated kinetic data for combustion modeling: supplement II. Journal of physical and chemical reference data 2005, 34, 757–1397
- Yelle \latinet al. 2006 Yelle, R. V.; Borggren, N.; de la Haye, V.; Kasprzak, W.; Niemann, H.; Müller-Wodarg, I.; Waite, J. The vertical structure of Titan’s upper atmosphere from Cassini Ion Neutral Mass Spectrometer measurements. Icarus 2006, 182, 567–576, Results from the Mars Express ASPERA-3 Investigation
- Strobel 2010 Strobel, D. F. Molecular hydrogen in Titan’s atmosphere: Implications of the measured tropospheric and thermospheric mole fractions. Icarus 2010, 208, 878–886
- Strobel 2012 Strobel, D. F. Hydrogen and methane in Titan’s atmosphere: Chemistry, diffusion, escape, and the Hunten limiting flux principle. Canadian Journal of Physics 2012, 90, 795–805
- Tucker \latinet al. 2013 Tucker, O.; Johnson, R.; Deighan, J.; Volkov, A. Diffusion and thermal escape of H2 from Titan’s atmosphere: Monte Carlo simulations. Icarus 2013, 222, 149–158
- Strobel 2022 Strobel, D. F. Molecular hydrogen in the upper atmospheres of Saturn and Titan. Icarus 2022, 376, 114876
- Tobie \latinet al. 2005 Tobie, G.; Grasset, O.; Lunine, J. I.; Mocquet, A.; Sotin, C. Titan’s internal structure inferred from a coupled thermal-orbital model. Icarus 2005, 175, 496–502
- Mandt \latinet al. 2009 Mandt, K. E.; Waite, J. H.; Lewis, W.; Magee, B.; Bell, J.; Lunine, J.; Mousis, O.; Cordier, D. Isotopic evolution of the major constituents of Titan’s atmosphere based on Cassini data. Planetary and Space Science 2009, 57, 1917–1930
- Mandt \latinet al. 2012 Mandt, K. E.; Waite, J. H.; Teolis, B. D.; Magee, B. A.; Bell, J. M.; Westlake, J. H.; Nixon, C. A.; Mousis, O.; Lunine, J. I. The 12C/13C ratio on Titan from Cassini INMS measurements and implications for the evolution of Titan’s methane. Astrophys. J. 2012, 749, 160
- Nixon \latinet al. 2012 Nixon, C. A.; Temelso, B.; Vinatier, S.; Teanby, N. A.; Bézard, B.; Achterberg, R. K.; Mandt, K. E.; Sherrill, C. D.; Irwin, P. G. J.; Jennings, D. E.; Romani, P. N.; Coustenis, A.; Flasar, F. M. Isotopic ratios in Titan’s methane: measurements and modeling. Astrophys. J. 2012, 749, 159
- Trafton 1972 Trafton, L. On the Possible Detection of H2 in Titan’s Atmosphere. The Astrophysical Journal 1972, 175, 285
- Courtin \latinet al. 1995 Courtin, R.; Gautier, D.; McKay, C. P. Titan’s Thermal Emission Spectrum: Reanalysis of the Voyager Infrared Measurements. Icarus 1995, 114, 144–162
- Courtin \latinet al. 2012 Courtin, R.; Sim, C. K.; Kim, S. J.; Gautier, D. The abundance of H2 in Titan’s troposphere from the Cassini CIRS investigation. Planetary and Space Science 2012, 69, 89–99
- 236 Strobel, D. F.; Cui, J. In Titan; Muller-Wodarg, I., Griffith, C. A., Lellouch, E., Cravens, T. E., Eds.; Cambridge University Press, pp 355–375
- McKay and Smith 2005 McKay, C.; Smith, H. Possibilities for methanogenic life in liquid methane on the surface of Titan. Icarus 2005, 178, 274–276
- Backx \latinet al. 1976 Backx, C.; Wight, G. R.; der Wiel, M. J. V. Oscillator strengths (10-70 eV) for absorption, ionization and dissociation in H2, HD and D2, obtained by an electron-ion coincidence method. Journal of Physics B: Atomic and Molecular Physics 1976, 9, 315–331
- McEwan and Anicich 2007 McEwan, M. J.; Anicich, V. G. Titan’s ion chemistry: A laboratory perspective. Mass spectrometry reviews 2007, 26, 281–319
- Espinosa-García and Corchado 1994 Espinosa-García, J.; Corchado, J. Variational transition-state theory calculation using the direct dynamics method: NH3+ H NH2+ H2 reaction. The Journal of chemical physics 1994, 101, 1333–1342
- Gerlich \latinet al. 2011 Gerlich, D.; Borodi, G.; Luca, A.; Mogo, C.; Smith, M. A. Reactions between cold CH and slow H and H2. Zeitschrift für Physikalische Chemie 2011, 225, 475–492
- Dutuit \latinet al. 2013 Dutuit, O.; Carrasco, N.; Thissen, R.; Vuitton, V.; Alcaraz, C.; Pernot, P.; Balucani, N.; Casavecchia, P.; Canosa, A.; Le Picard, S., \latinet al. Critical review of N, N+, N, N++, and N main production processes and reactions of relevance to Titan’s atmosphere. The Astrophysical Journal Supplement Series 2013, 204, 20
- Brownsword \latinet al. 1997 Brownsword, R. A.; Canosa, A.; Rowe, B. R.; Sims, I. R.; Smith, I. W.; Stewart, D. W.; Symonds, A. C.; Travers, D. Kinetics over a wide range of temperature (13–744 K): rate constants for the reactions of CH (= 0) with H2 and D2 and for the removal of CH (= 1) by H2 and D2. The Journal of chemical physics 1997, 106, 7662–7677
- Brownsword \latinet al. 1997 Brownsword, R. A.; Sims, I. R.; Smith, I. W.; Stewart, D. W.; Canosa, A.; Rowe, B. R. The Radiative Association of CH with H2: A Mechanism for formation of CH3 in Interstellar Clouds. The Astrophysical Journal 1997, 485, 195
- Klippenstein \latinet al. 2006 Klippenstein, S. J.; Georgievskii, Y.; Harding, L. B. Predictive theory for the combination kinetics of two alkyl radicals. Physical Chemistry Chemical Physics 2006, 8, 1133–1147
- Kuiper 1944 Kuiper, G. P. Titan: a Satellite with an Atmosphere. Astrophys. J. 1944, 100, 378–+
- Gans \latinet al. 2011 Gans, B.; Boyé-Péronne, S.; Broquier, M.; Delsaut, M.; Douin, S.; Fellows, C. E.; Halvick, P.; Loison, J.-C.; Lucchese, R. R.; Gauyacq, D. Photolysis of methane revisited at 121.6 nm and at 118.2 nm: quantum yields of the primary products, measured by mass spectrometry. Physical Chemistry Chemical Physics 2011, 13, 8140
- Gans \latinet al. 2013 Gans, B.; Peng, Z.; Carrasco, N.; Gauyacq, D.; Lebonnois, S.; Pernot, P. Impact of a new wavelength-dependent representation of methane photolysis branching ratios on the modeling of Titan’s atmospheric photochemistry. Icarus 2013, 223, 330–343
- Lorenz \latinet al. 1997 Lorenz, R. D.; McKay, C. P.; Lunine, J. I. Photochemically-induced collapse of Titan’s atmosphere. Science 1997, 275, 642–644
- Wong \latinet al. 2015 Wong, M. L.; Yung, Y. L.; Randall Gladstone, G. Pluto’s implications for a Snowball Titan. Icarus 2015, 246, 192–196, Special Issue: The Pluto System
- Tobie \latinet al. 2006 Tobie, G.; Lunine, J. I.; Sotin, C. Episodic outgassing as the origin of atmospheric methane on Titan. Nature 2006, 440, 61–64
- Fortes \latinet al. 2007 Fortes, A. D.; Grindrod, P. M.; Trickett, S. K.; Vočadlo, L. Ammonium sulfate on Titan: Possible origin and role in cryovolcanism. Icarus 2007, 188, 139–153
- Lopes \latinet al. 2013 Lopes, R. M. C. \latinet al. Cryovolcanism on Titan: New results from Cassini RADAR and VIMS. Journal of Geophysical Research (Planets) 2013, 118, 416–435
- Sohl \latinet al. 2014 Sohl, F.; Solomonidou, A.; Wagner, F.; Coustenis, A.; Hussmann, H.; Schulze-Makuch, D. Structural and tidal models of Titan and inferences on cryovolcanism. Journal of Geophysical Research: Planets 2014, 119, 1013–1036
- Choukroun and Sotin 2012 Choukroun, M.; Sotin, C. Is Titan’s shape caused by its meteorology and carbon cycle? Geophys. Res. Lett. 2012, 39, L04201
- Lellouch \latinet al. 2014 Lellouch, E.; Bézard, B.; Flasar, F. M.; Vinatier, S.; Achterberg, R.; Nixon, C. A.; Bjoraker, G. L.; Gorius, N. The distribution of methane in Titan’s stratosphere from Cassini/CIRS observations. Icarus 2014, 231, 323–337
- Hébrard \latinet al. 2006 Hébrard, E.; Dobrijevic, M.; Bénilan, Y.; Raulin, F. Photochemical kinetics uncertainties in modeling Titan’s atmosphere: A review. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 2006, 7, 211–230
- Fleurat-Lessard \latinet al. 2002 Fleurat-Lessard, P.; Rayez, J.-C.; Bergeat, A.; Loison, J.-C. Reaction of methylidyne CH (X2) radical with CH4 and H2S: Overall rate constant and absolute atomic hydrogen production. Chemical physics 2002, 279, 87–99
- Gillett 1975 Gillett, F. C. Further observations of the 8–13 m spectrum of Titan. The Astrophysical Journal 1975, 201, L41
- Läuter \latinet al. 2002 Läuter, A.; Lee, K.; Jung, K.; Vatsa, R.; Mittal, J.; Volpp, H.-R. Absolute primary H atom quantum yield measurements in the 193.3 and 121.6 nm photodissociation of acetylene. Chem. Phys. Lett. 2002, 358, 314–319
- Kovács \latinet al. 2010 Kovács, T.; Blitz, M. A.; Seakins, P. W. H-atom Yields from the Photolysis of Acetylene and from the Reaction of C2H with H2, C2H2 and C2H4. J. Phys. Chem. A 2010, 114, 4735–4741
- Jasper \latinet al. 2007 Jasper, A. W.; Klippenstein, S. J.; Harding, L. B. Secondary kinetics of methanol decomposition: Theoretical rate coefficients for 3CH2+ OH, 3CH2+ 3CH2, and 3CH2+ CH3. The Journal of Physical Chemistry A 2007, 111, 8699–8707
- Holland \latinet al. 1997 Holland, D.; Shaw, D.; Hayes, M.; Shpinkova, L.; Rennie, E.; Karlsson, L.; Baltzer, P.; Wannberg, B. A photoabsorption, photodissociation and photoelectron spectroscopy study of C2H4 and C2D4. Chemical physics 1997, 219, 91–116
- Ehlerding \latinet al. 2004 Ehlerding, A.; Hellberg, F.; Thomas, R.; Kalhori, S.; Viggiano, A. A.; Arnold, S. T.; Larsson, M.; Af Ugglas, M. Dissociative recombination of C2H+ and C2H: Absolute cross sections and product branching ratios. Physical Chemistry Chemical Physics 2004, 6, 949–954
- Kalhori \latinet al. 2002 Kalhori, S.; Viggiano, A.; Arnold, S.; Rosén, S.; Semaniak, J.; Derkatch, A.; Larsson, M. Dissociative recombination of C2H. Astronomy & Astrophysics 2002, 391, 1159–1165
- Janev and Reiter 2004 Janev, R. K.; Reiter, D. Collision processes of C2,3Hy and C2,3H hydrocarbons with electrons and protons. Physics of Plasmas 2004, 11, 780–829
- Chabot \latinet al. 2013 Chabot, M.; Béroff, K.; Gratier, P.; Jallat, A.; Wakelam, V. Reactions Forming C, Cn=2,4H(0,+), and C3H in the Gas Phase: Semiempirical Branching Ratios. The Astrophysical Journal 2013, 771, 90
- Gannon \latinet al. 2010 Gannon, K. L.; Blitz, M. A.; Liang, C.-H.; Pilling, M. J.; Seakins, P. W.; Glowacki, D. R.; Harvey, J. N. An experimental and theoretical investigation of the competition between chemical reaction and relaxation for the reactions of 1CH2 with acetylene and ethene: implications for the chemistry of the giant planets. Faraday discussions 2010, 147, 173–188
- Gannon \latinet al. 2010 Gannon, K.; Blitz, M.; Liang, C.; Pilling, M.; Seakins, P.; Glowacki, D. Temperature dependent kinetics (195- 798 K) and H atom yields (298- 498 K) from reactions of 1CH2 with acetylene, ethene, and propene. The Journal of Physical Chemistry A 2010, 114, 9413–9424
- Gannon \latinet al. 2010 Gannon, K.; Blitz, M.; Kovacs, T.; Pilling, M.; Seakins, P. State resolved measurements of a 1CH2 removal confirm predictions of the gateway model for electronic quenching. The Journal of chemical physics 2010, 132, 024302
- Chastaing \latinet al. 1998 Chastaing, D.; James, P. L.; Sims, I. R.; Smith, I. W. Neutral–neutral reactions at the temperatures of interstellar clouds Rate coefficients for reactions of C2H radicals with O2, C2H2, C2H4 and C3H6 down to 15 K. Faraday Discussions 1998, 109, 165–181
- Singh \latinet al. 2016 Singh, S.; McCord, T. B.; Combe, J.-P.; Rodriguez, S.; Maltagliati, L.; Cornet, T.; Mouélic, S. L.; Clark, R. N.; Chevrier, E.-m. s., V. F. Acetylene on Titan’s Surface. Astrophys. J. 2016, 828
- Cordier \latinet al. 2009 Cordier, D.; Mousis, O.; Lunine, J. I.; Lavvas, P.; Vuitton, V. An Estimate of the Chemical Composition of Titan’s Lakes. The Astrophysical Journal 2009, 707, L128–L131
- Cordier \latinet al. 2012 Cordier, D.; Mousis, O.; Lunine, J.; Lebonnois, S.; Rannou, P.; Lavvas, P.; Lobo, L.; Ferreira, A. Titan’s lakes chemical composition: Sources of uncertainties and variability. Planetary and Space Science 2012, 61, 99–107, Surfaces, atmospheres and magnetospheres of the outer planets and their satellites and ring systems: Part VII
- Barnes \latinet al. 2021 Barnes, J. W. \latinet al. Science Goals and Objectives for the Dragonfly Titan Rotorcraft Relocatable Lander. The Planetary Science Journal 2021, 2, 130
- Cable \latinet al. 2018 Cable, M. L.; Vu, T. H.; Maynard-Casely, H. E.; Choukroun, M.; Hodyss, R. The Acetylene-Ammonia Co-crystal on Titan. ACS Earth and Space Chemistry 2018, 2, 366–375
- Cable \latinet al. 2019 Cable, M. L.; Vu, T. H.; Malaska, M. J.; Maynard-Casely, H. E.; Choukroun, M.; Hodyss, R. A Co-Crystal between Acetylene and Butane: A Potentially Ubiquitous Molecular Mineral on Titan. ACS Earth and Space Chemistry 2019, 3, 2808–2815
- Vinatier \latinet al. 2010 Vinatier, S.; Bézard, B.; Nixon, C. A.; Mamoutkine, A.; Carlson, R. C.; Jennings, D. E.; Guandique, E. A.; Teanby, N. A.; Bjoraker, G. L.; Michael Flasar, F.; Kunde, V. G. Analysis of Cassini/CIRS limb spectra of Titan acquired during the nominal mission. I. Hydrocarbons, nitriles and CO2 vertical mixing ratio profiles. Icarus 2010, 205, 559–570
- Balko \latinet al. 1992 Balko, B. A.; Zhang, J.; Lee, Y. T. Photodissociation of ethylene at 193 nm. The Journal of Chemical Physics 1992, 97, 935–942
- Lee \latinet al. 2004 Lee, S.-H.; Lee, Y. T.; Yang, X. Dynamics of photodissociation of ethylene and its isotopomers at 157 nm: Branching ratios and kinetic-energy distributions. The Journal of Chemical Physics 2004, 120, 10983–10991
- McLain \latinet al. 2004 McLain, J. L.; Poterya, V.; Molek, C. D.; Babcock, L. M.; Adams, N. G. Flowing afterglow studies of the temperature dependencies for dissociative recombination of O, CH, C2H, and C6H with electrons. The Journal of Physical Chemistry A 2004, 108, 6704–6708
- Geppert \latinet al. 2004 Geppert, W.; Ehlerding, A.; Hellberg, F.; Kalhori, S.; Thomas, R.; Novotny, O.; Arnold, S.; Miller, T.; Viggiano, A.; Larsson, M. First Observation of Four-Body Breakup in Electron Recombination: C2D. Physical review letters 2004, 93, 153201
- Canosa \latinet al. 1997 Canosa, A.; Sims, I. R.; Travers, D.; Smith, I. W. M.; Rowe, B. R. Reactions of the methylidine radical with CH4, C2H2, C2H4, C2H6, and but-1-ene studied between 23 and 295K with a CRESU apparatus. å 1997, 323, 644–651
- Goulay \latinet al. 2009 Goulay, F.; Trevitt, A. J.; Meloni, G.; Selby, T. M.; Osborn, D. L.; Taatjes, C. A.; Vereecken, L.; Leone, S. R. Cyclic versus linear isomers produced by reaction of the methylidyne radical (CH) with small unsaturated hydrocarbons. Journal of the American Chemical Society 2009, 131, 993–1005
- Gillett \latinet al. 1973 Gillett, F. C.; Forrest, W. J.; Merrill, K. M. 8-13 m Observations of Titan. The Astrophysical Journal 1973, 184, L93
- Coustenis \latinet al. 2007 Coustenis, A. \latinet al. The composition of Titan’s stratosphere from Cassini/CIRS mid-infrared spectra. Icarus 2007, 189, 35–62
- Coustenis \latinet al. 2010 Coustenis, A.; Jennings, D. E.; Nixon, C. A.; Achterberg, R. K.; Lavvas, P.; Vinatier, S.; Teanby, N. A.; Bjoraker, G. L.; Carlson, R. C.; Piani, L.; Bampasidis, G.; Flasar, F. M.; Romani, P. N. Titan trace gaseous composition from CIRS at the end of the Cassini-Huygens prime mission. Icarus 2010, 207, 461–476
- Lunine \latinet al. 1983 Lunine, J. I.; Stevenson, D. J.; Yung, Y. L. Ethane ocean on Titan. Science 1983, 222, 1229–+
- Flasar 1983 Flasar, F. M. Oceans on Titan? Science 1983, 221, 55–57
- Muhleman \latinet al. 1990 Muhleman, D. O.; Grossman, A. W.; Butler, B. J.; Slade, M. A. Radar Reflectivity of Titan. Science 1990, 248, 975–980
- Smith \latinet al. 1996 Smith, P. H.; Lemmon, M. T.; Lorenz, R. D.; Sromovsky, L. A.; Caldwell, J. J.; Allison, M. D. Titan’s Surface, Revealed by HST Imaging. Icarus 1996, 119, 336–349
- Stofan \latinet al. 2007 Stofan, E. R. \latinet al. The lakes of Titan. Nature 2007, 445, 61–64
- Gall \latinet al. 2016 Gall, A. L.; Malaska, M. J.; Lorenz, R. D.; Janssen, M. A.; Tokano, T.; Hayes, A. G.; Mastrogiuseppe, M.; Lunine, J. I.; Veyssière, G.; Encrenaz, P.; Karatekin, O. Composition, seasonal change, and bathymetry of Ligeia Mare, Titan, derived from its microwave thermal emission. Journal of Geophysical Research: Planets 2016, 121, 233–251
- Mastrogiuseppe \latinet al. 2018 Mastrogiuseppe, M.; Poggiali, V.; Hayes, A.; Lunine, J.; Seu, R.; Di Achille, G.; Lorenz, R. Cassini radar observation of Punga Mare and environs: Bathymetry and composition. Earth and Planetary Science Letters 2018, 496, 89–95
- Brown \latinet al. 2008 Brown, R. H.; Soderblom, L. A.; Soderblom, J. M.; Clark, R. N.; Jaumann, R.; Barnes, J. W.; Sotin, C.; Buratti, B.; Baines, K. H.; Nicholson, P. D. The identification of liquid ethane in Titan’s Ontario Lacus. Nature 2008, 454, 607–610
- Mastrogiuseppe \latinet al. 2018 Mastrogiuseppe, M.; Hayes, A.; Poggiali, V.; Lunine, J.; Lorenz, R.; Seu, R.; Le Gall, A.; Notarnicola, C.; Mitchell, K.; Malaska, M.; Birch, S. Bathymetry and composition of Titan’s Ontario Lacus derived from Monte Carlo-based waveform inversion of Cassini RADAR altimetry data. Icarus 2018, 300, 203–209
- Cable \latinet al. 2014 Cable, M. L.; Vu, T. H.; Hodyss, R.; Choukroun, M.; Malaska, M. J.; Beauchamp, P. Experimental determination of the kinetics of formation of the benzene-ethane co-crystal and implications for Titan. Geophysical Research Letters 2014, 41, 5396–5401
- Wilson and Atreya 2009 Wilson, E. H.; Atreya, S. K. Titan’s Carbon Budget and the Case of the Missing Ethane. Journal of Physical Chemistry A 2009, 113, 11221–11226
- Laufer and Fahr 2004 Laufer, A. H.; Fahr, A. Reactions and kinetics of unsaturated C2 hydrocarbon radicals. Chemical reviews 2004, 104, 2813–2832
- Akimoto \latinet al. 1965 Akimoto, H.; Obi, K.; Tanaka, I. Primary Process in the Photolysis of Ethane at 1236 . The Journal of Chemical Physics 1965, 42, 3864–3868
- Hampson and McNesby 1965 Hampson, R. F.; McNesby, J. R. Vacuum-Ultraviolet Photolysis of Ethane at High Temperature. The Journal of Chemical Physics 1965, 42, 2200–2208
- Lias \latinet al. 1970 Lias, S. G.; Collin, G. J.; Rebbert, R. E.; Ausloos, P. Photolysis of Ethane at 11.6–11.8 eV. The Journal of Chemical Physics 1970, 52, 1841–1851
- Cordier \latinet al. 2013 Cordier, D.; Mousis, O.; Lunine, J. I.; Lavvas, P.; Vuitton, V. ERRATUM: An Estimate of the Chemical Composition of Titan’s Lakes (2009, ApJL, 707, L128). The Astrophysical Journal 2013, 768, L23
- Griffith \latinet al. 2006 Griffith, C. A.; Penteado, P.; Rannou, P.; Brown, R.; Boudon, V.; Baines, K. H.; Clark, R.; Drossart, P.; Buratti, B.; Nicholson, P.; McKay, C. P.; Coustenis, A.; Negrao, A.; Jaumann, R. Evidence for a Polar Ethane Cloud on Titan. Science 2006, 313, 1620–1622
- Anderson \latinet al. 2014 Anderson, C. M.; Samuelson, R.; Achterberg, R.; Barnes, J.; Flasar, F. Subsidence-induced methane clouds in Titan’s winter polar stratosphere and upper troposphere. Icarus 2014, 243, 129–138
- Thaddeus \latinet al. 1985 Thaddeus, P.; Vrtilek, J. M.; Gottlieb, C. A. Laboratory and astronomical identification of cyclopropenylidene, C3H2. The Astrophysical Journal 1985, 299, L63
- Walch 1995 Walch, S. P. Characterization of the minimum energy paths for the reactions of CH() and with . The Journal of Chemical Physics 1995, 103, 7064–7071
- Guadagnini \latinet al. 1998 Guadagnini, R.; Schatz, G. C.; Walch, S. P. Ab Initio and RRKM Studies of the Reactions of C, CH, and 1CH2 with Acetylene. The Journal of Physical Chemistry A 1998, 102, 5857–5866
- Seburg and Squires 1997 Seburg, R. A.; Squires, R. R. The electron affinity of cyclopropyl radical measured by the kinetic method. International Journal of Mass Spectrometry and Ion Processes 1997, 167-168, 541–557, In Honour of Chava Lifshitz
- Wu \latinet al. 2010 Wu, Q.; Cheng, Q.; Yamaguchi, Y.; Li, Q.; Schaefer, H. F. Triplet states of cyclopropenylidene and its isomers. The Journal of Chemical Physics 2010, 132, 044308
- Willacy \latinet al. 2022 Willacy, K.; Chen, S.; Adams, D. J.; Yung, Y. L. Vertical distribution of cyclopropenylidene and propadiene in the atmosphere of Titan. arXiv preprint arXiv:2204.13064 2022,
- Prodnuk \latinet al. 1992 Prodnuk, S. D.; Grocert, S.; Bierbaum, V. M.; DePuy, C. H. Gas-phase reactions of C3Hn+ ions. Organic mass spectrometry 1992, 27, 416–422
- Poterya \latinet al. 2005 Poterya, V.; McLain, J. L.; Adams, N. G.; Babcock, L. M. Mechanisms of electron-ion recombination of N2H+/N2D+ and HCO+/DCO+ ions: temperature dependence and isotopic effect. The Journal of Physical Chemistry A 2005, 109, 7181–7186
- Cernicharo \latinet al. 1991 Cernicharo, J.; Gottlieb, C. A.; Guelin, M.; Killian, T. C.; Paubert, G.; Thaddeus, P.; Vrtilek, J. M. Astronomical detection of H2CCC. The Astrophysical Journal 1991, 368, L39
- Hanel \latinet al. 1980 Hanel, R.; Crosby, D.; Herath, L.; Vanous, D.; Collins, D.; Creswick, H.; Harris, C.; Rhodes, M. Infrared spectrometer for Voyager. Applied Optics 1980, 19, 1391
- Maguire \latinet al. 1981 Maguire, W. C.; Hanel, R. A.; Jennings, D. E.; Kunde, V. G.; Samuelson, R. E. C3H8 and C3H4 in Titan’s atmosphere. Nature 1981, 292, 683–686
- Seki and Okabe 1992 Seki, K.; Okabe, H. Photodissociation of methylacetylene at 193 nm. Journal of Physical Chemistry 1992, 96
- Ni \latinet al. 1999 Ni, C.-K.; Huang, J. D.; Chen, Y. T.; Kung, A. H.; Jackson, W. M. Photodissociation of propyne and allene at 193 nm with vacuum ultraviolet detection of the products. The Journal of Chemical Physics 1999, 110, 3320–3325
- DeSain and Taatjes 2003 DeSain, J. D.; Taatjes, C. A. Infrared Laser Absorption Measurements of the Kinetics of Propargyl Radical Self-Reaction and the 193 nm Photolysis of Propyne. The Journal of Physical Chemistry A 2003, 107, 4843–4850
- Chastaing \latinet al. 2001 Chastaing, D.; Le Picard, S.; Sims, I.; Smith, I. Rate coefficients for the reactions of C (P) atoms with C2H2, C2H4, CH3CCH and H2CCCH2 at temperatures down to 15 K. Astronomy & Astrophysics 2001, 365, 241–247
- Collin 1988 Collin, G. Photochemistry of simple olefins: Chemistry of electronic excited states or hot ground state? Adv. Photochemistry 1988, 14, 135–176
- Houriet \latinet al. 1978 Houriet, R.; Elwood, T. A.; Futrell, J. H. A tandem ion cyclotron resonance study of the reactions of allyl ions with benzene and substituted benzene. Journal of the American Chemical Society 1978, 100, 2320–2324
- Edwards \latinet al. 2008 Edwards, S. J.; Freeman, C. G.; McEwan, M. J. The ion chemistry of methylenimine and propionitrile and their relevance to Titan. International Journal of Mass Spectrometry 2008, 272, 86–90
- Fahr and Nayak 1996 Fahr, A.; Nayak, A. Temperature dependent ultraviolet absorption cross sections of propylene, methylacetylene and vinylacetylene. Chemical physics 1996, 203, 351–358
- Lombardo \latinet al. 2019 Lombardo, N. A.; Nixon, C. A.; Achterberg, R. K.; Jolly, A.; Sung, K.; Irwin, P. G. J.; Flasar, F. M. Spatial and seasonal variations in C3Hx hydrocarbon abundance in Titan’s stratosphere from Cassini CIRS observations. Icarus 2019, 317, 454–469
- Jackson \latinet al. 1991 Jackson, W. M.; Anex, D. S.; Continetti, R. E.; Balko, B. A.; Lee, Y. T. Molecular beam studies of the photolysis of allene and the secondary photodissociation of the C3Hx fragments. The Journal of Chemical Physics 1991, 95, 7327–7336
- Harich \latinet al. 2000 Harich, S.; Lee, Y. T.; Yang, X. Photodissociation dynamics of allene at 157 nm. Physical Chemistry Chemical Physics 2000, 2, 1187–1191
- Robinson \latinet al. 2005 Robinson, J. C.; Sveum, N. E.; Goncher, S. J.; Neumark, D. M. Photofragment translational spectroscopy of allene, propyne, and propyne-d3 at 193 nm. Molecular Physics 2005, 103, 1765–1783
- Gierczak \latinet al. 1988 Gierczak, T.; Gawlowski, J.; Niedzielski, J. Reactions of excited C3H5 radicals: Implications for the photolysis of propylene at 8.4 eV. Journal of Photochemistry and Photobiology A: Chemistry 1988, 43, 1–9
- Nixon \latinet al. 2013 Nixon, C. A.; Jennings, D. E.; Bézard, B.; Vinatier, S.; Teanby, N. A.; Sung, K.; Ansty, T. M.; Irwin, P. G. J.; Gorius, N.; Cottini, V.; Coustenis, A.; Flasar, F. M. Detection of Propene in Titan’s Stratosphere. Astrophys. J. Lett. 2013, 776, L14
- Borrell \latinet al. 1971 Borrell, P.; Cervenka, A.; Turner, J. W. Pressure effects and quantum yields in the photolysis of ethylene and propene at 185 nm. Journal of the Chemical Society B: Physical Organic 1971, 2293
- Collin \latinet al. 1979 Collin, G. J.; Deslauriers, H.; Deschênes, J. Photolyse du propène et du méthyl-2-butène-2 vers 174 et à 163 nm. Canadian Journal of Chemistry 1979, 57, 870–875
- Niedzielski \latinet al. 1982 Niedzielski, J.; Makulski, W.; Gawłowski, J. Gas phase photolysis of propylene at 8.4 and 10.0 eV. Journal of Photochemistry 1982, 19, 123–131
- Narożnik and Niedzielski 1986 Narożnik, M.; Niedzielski, J. Propylene photolysis at 6.7 eV: calculation of the quantum yields for the secondary processes. Journal of Photochemistry 1986, 32, 281–292
- Roe \latinet al. 2003 Roe, H. G.; Greathouse, T.; Richter, M.; Lacy, J. Propane on Titan. The Astrophysical Journal 2003, 597, L65
- Nixon \latinet al. 2009 Nixon, C. A.; Jennings, D. E.; Flaud, J.-M.; Bézard, B.; Teanby, N. A.; Irwin, P. G. J.; Ansty, T. M.; Coustenis, A.; Vinatier, S.; Flasar, F. M. Titan’s prolific propane: The Cassini CIRS perspective. Plan. & Space Sci. 2009, 57, 1573–1585
- Okabe and McNesby 1962 Okabe, H.; McNesby, J. R. Vacuum Ultraviolet Photochemistry. IV. Photolysis of Propane. The Journal of Chemical Physics 1962, 37, 1340–1346
- Laufer and McNesby 1966 Laufer, A.; McNesby, J. The chain decomposition of propane initiated by vacuum ultraviolet photolysis. J. Phys. Chem. 1966, 70, 4094–4096
- Harding \latinet al. 2007 Harding, L. B.; Klippenstein, S. J.; Georgievskii, Y. On the combination reactions of hydrogen atoms with resonance-stabilized hydrocarbon radicals. The Journal of Physical Chemistry A 2007, 111, 3789–3801
- Murphy \latinet al. 2003 Murphy, J. E.; Vakhtin, A. B.; Leone, S. R. Laboratory kinetics of C2H radical reactions with ethane, propane, and n-butane at T= 96–296 K: implications for Titan. Icarus 2003, 163, 175–181
- Sung \latinet al. 2013 Sung, K.; Toon, G. C.; Mantz, A. W.; Smith, M. A. H. FT-IR measurements of cold C3H8 cross sections at 7—15 m for Titan atmosphere. Icarus 2013,
- Kunde \latinet al. 1981 Kunde, V. G.; Aikin, A. C.; Hanel, R. A.; Jennings, D. E.; Maguire, W. C.; Samuelson, R. E. C4H2, HC3N and C2N2 in Titan’s atmosphere. Nature 1981, 292, 686–688
- Vereecken and Peeters 1999 Vereecken, L.; Peeters, J. Detailed microvariational RRKM master equation analysis of the product distribution of the C2H2 + CH (X) reaction over extended temperature and pressure ranges. The Journal of Physical Chemistry A 1999, 103, 5523–5533
- Silva \latinet al. 2008 Silva, R.; Gichuhi, W.; Huang, C.; Doyle, M.; Kislov, V.; Mebel, A.; Suits, A. H elimination and metastable lifetimes in the UV photoexcitation of diacetylene. PNAS; Proceedings of the National Academy of Sciences 2008, 105, 12713–12718
- May 2008 May, O. Absolute cross sections for dissociative electron attachment to acetylene and diacetylene. Physical Review A 2008, 77
- Nahar and Pradhan 1997 Nahar, S. N.; Pradhan, A. K. Electron-ion recombination rate coefficients, photoionization cross sections, and ionization fractions for astrophysically abundant elements. I. Carbon and nitrogen. The Astrophysical Journal Supplement Series 1997, 111, 339
- Gu \latinet al. 2009 Gu, X.; Kim, Y.; Kaiser, R.; Mebel, A.; Liang, M.; Yung, Y. Chemical dynamics of triacetylene formation and implications to the synthesis of polyynes in Titan’s atmosphere. Proceedings of the National Academy of Sciences 2009, 106, 16078–16083
- Cernicharo \latinet al. 2001 Cernicharo, J.; Heras, A.; Tielens, A.; Pardo, N.; Herpin, F.; Guelin, M.; Waters, L. Infrared Space Observatory’s discovery of C4H2, C6H2, and benzene in CRL 618. The Astrophysical Journal 2001, 546, L123–L126
- Tsai \latinet al. 2000 Tsai, S.-T.; Lin, C.-K.; Lee, Y. T.; Ni, C.-K. Dissociation rate of hot benzene. The Journal of Chemical Physics 2000, 113, 67–70
- Kislov \latinet al. 2004 Kislov, V. V.; Nguyen, T. L.; Mebel, A. M.; Lin, S. H.; Smith, S. C. Photodissociation of benzene under collision-free conditions: An ab initio/Rice–Ramsperger–Kassel–Marcus study. The Journal of Chemical Physics 2004, 120, 7008–7017
- Ni and Lee 2004 Ni, C.-K.; Lee, Y. T. Photodissociation of simple aromatic molecules in a molecular beam. International Reviews in Physical Chemistry 2004, 23, 187–218
- Yokoyama \latinet al. 1990 Yokoyama, A.; Zhao, X.; Hintsa, E. J.; Continetti, R. E.; Lee, Y. T. Molecular beam studies of the photodissociation of benzene at 193 and 248 nm. The Journal of Chemical Physics 1990, 92, 4222–4233
- Kaiser and Hansen 2021 Kaiser, R. I.; Hansen, N. An Aromatic Universe- A Physical Chemistry Perspective. The Journal of Physical Chemistry A 2021, 125, 3826–3840
- Kaiser \latinet al. 2021 Kaiser, R. I.; Zhao, L.; Lu, W.; Ahmed, M.; Zagidullin, M. V.; Azyazov, V. N.; Mebel, A. M. Formation of Benzene and Naphthalene through Cyclopentadienyl-Mediated Radical–Radical Reactions. The Journal of Physical Chemistry Letters 2021, 13, 208–213
- Hamberg \latinet al. 2011 Hamberg, M.; Vigren, E.; Thomas, R.; Zhaunerchyk, V.; Zhang, M.; Trippel, S.; Kaminska, M.; Kashperka, I.; af Ugglas, M.; Kallberg, A., \latinet al. PAHs and the Universe; EDP Sciences, 2011; pp 241–250
- Jones \latinet al. 2011 Jones, B. M.; Zhang, F.; Kaiser, R. I.; Jamal, A.; Mebel, A. M.; Cordiner, M. A.; Charnley, S. B. Formation of benzene in the interstellar medium. Proceedings of the National Academy of Sciences 2011, 108, 452–457
- Capalbo \latinet al. 2016 Capalbo, F. J.; Bénilan, Y.; Fray, N.; Schwell, M.; Champion, N.; touhami Es-sebbar, E.; Koskinen, T. T.; Lehocki, I.; Yelle, R. V. New benzene absorption cross sections in the VUV, relevance for Titan’s upper atmosphere. Icarus 2016, 265, 95–109
- Gu and Kaiser 2009 Gu, X.; Kaiser, R. I. Reaction dynamics of phenyl radicals in extreme environments: a crossed molecular beam study. Accounts of chemical research 2009, 42, 290–302
- Cordiner \latinet al. 2014 Cordiner, M. A.; Nixon, C. A.; Teanby, N. A.; Irwin, P. G. J.; Serigano, J.; Charnley, S. B.; Milam, S. N.; Mumma, M. J.; Lis, D. C.; Villanueva, G.; Paganini, L.; Kuan, Y.-J.; Remijan, A. J. ALMA Measurements of the HNC and HC3N Distributions in Titan’s Atmosphere. Astrophys. J. Lett. 2014, 795, L30
- Trafton 1972 Trafton, L. The Bulk Composition of Titan’s Atmosphere. Astrophys. J. 1972, 175, 295–306
- Hunten 1973 Hunten, D. M. The Escape of H2 from Titan. Journal of the Atmospheric Sciences 1973, 30, 726–732
- Broadfoot \latinet al. 1981 Broadfoot, A. \latinet al. Extreme Ultraviolet Observations from Voyager 1 Encounter with Saturn. Science 1981, 212, 206–211
- Vervack \latinet al. 2004 Vervack, R. J.; Sandel, B. R.; Strobel, D. F. New perspectives on Titan’s upper atmosphere from a reanalysis of the Voyager 1 UVS solar occultations. Icarus 2004, 170, 91–112
- Ajello \latinet al. 2007 Ajello, J. M.; Stevens, M. H.; Stewart, I.; Larsen, K.; Esposito, L.; Colwell, J.; McClintock, W.; Holsclaw, G.; Gustin, J.; Pryor, W. Titan airglow spectra from Cassini Ultraviolet Imaging Spectrograph (UVIS): EUV analysis. Geophysical Research Letters 2007, 34
- Ajello \latinet al. 2008 Ajello, J. M.; Gustin, J.; Stewart, I.; Larsen, K.; Esposito, L.; Pryor, W.; McClintock, W.; Stevens, M. H.; Malone, C. P.; Dziczek, D. Titan airglow spectra from the Cassini Ultraviolet Imaging Spectrograph: FUV disk analysis. Geophysical Research Letters 2008, 35
- Stevens \latinet al. 2011 Stevens, M. H.; Gustin, J.; Ajello, J. M.; Evans, J. S.; Meier, R. R.; Kochenash, A. J.; Stephan, A. W.; Stewart, A. I. F.; Esposito, L. W.; McClintock, W. E.; Holsclaw, G.; Bradley, E. T.; Lewis, B. R.; Heays, A. N. The production of Titan's ultraviolet nitrogen airglow. Journal of Geophysical Research: Space Physics 2011, 116
- Capalbo \latinet al. 2013 Capalbo, F. J.; Bénilan, Y.; Yelle, R. V.; Koskinen, T. T.; Sandel, B. R.; Holsclaw, G. M.; McClintock, W. E. Solar Occulation by Titan Measured by Cassini UVIS. The Astrophysical Journal 2013, 766, L16
- Owen 1982 Owen, T. The atmosphere of Titan. Journal of Molecular Evolution 1982, 18, 150–156
- Atreya \latinet al. 1978 Atreya, S. K.; Donahue, T. M.; Kuhn, W. R. Evolution of a Nitrogen Atmosphere on Titan. Science 1978, 201, 611–613
- McKay \latinet al. 1988 McKay, C. P.; Scattergood, T. W.; Pollack, J. B.; Borucki, W. J.; van Ghyseghem, H. T. High-temperature shock formation of N2 and organics on primordial Titan. Nature 1988, 332, 520–522
- Fukuzaki \latinet al. 2010 Fukuzaki, S.; Sekine, Y.; Genda, H.; Sugita, S.; Kadono, T.; Matsui, T. Impact-induced N2 production from ammonium sulfate: Implications for the origin and evolution of N2 in Titan’s atmosphere. Icarus 2010, 209, 715–722
- Xu \latinet al. 2013 Xu, Y.; Chang, Y. C.; Lu, Z.; Ng, C. Absolute Integral Cross Sections and Product Branching Ratios for the Vibrationally Selected ion-molecule reactions: N (; = 0–2)+ CH4. The Astrophysical Journal 2013, 769, 72
- Wakelam \latinet al. 2015 Wakelam, V.; Loison, J.-C.; Herbst, E.; Pavone, B.; Bergeat, A.; Béroff, K.; Chabot, M.; Faure, A.; Galli, D.; Geppert, W. D., \latinet al. The 2014 KIDA network for interstellar chemistry. The Astrophysical Journal Supplement Series 2015, 217, 20
- Lavvas \latinet al. 2011 Lavvas, P.; Sander, M.; Kraft, M.; Imanaka, H. Surface Chemistry and Particle Shape: Processes for the Evolution of Aerosols in Titan’s Atmosphere. The Astrophysical Journal 2011, 728, 80
- Lavvas \latinet al. 2015 Lavvas, P.; Yelle, R.; Heays, A.; Campbell, L.; Brunger, M.; Galand, M.; Vuitton, V. N2 state population in Titan’s atmosphere. Icarus 2015, 260, 29–59
- Marquette \latinet al. 1988 Marquette, J.; Rebrion, C.; Rowe, B. Reactions of N+(3P) ions with normal, para, and deuterated hydrogens at low temperatures. The Journal of chemical physics 1988, 89, 2041–2047
- Liang \latinet al. 2007 Liang, M.; Heays, A. N.; Lewis, B. R.; Gibson, S. T.; Yung, Y. L. Source of Nitrogen Isotope Anomaly in HCN in the Atmosphere of Titan. Astrophys. J. Lett. 2007, 664, L115–L118
- Vinatier \latinet al. 2007 Vinatier, S.; Bézard, B.; Nixon, C. A. The Titan 14N/15N and 12C/13C isotopic ratios in HCN from Cassini/CIRS. Icarus 2007, 191, 712–721
- Jennings \latinet al. 2008 Jennings, D. E.; Nixon, C. A.; Jolly, A.; Bézard, B.; Coustenis, A.; Vinatier, S.; Irwin, P. G. J.; Teanby, N. A.; Romani, P. N.; Achterberg, R. K.; Flasar, F. M. Isotopic Ratios in Titan’s Atmosphere from Cassini CIRS Limb Sounding: HC3N in the North. Astrophys. J. Lett. 2008, 681, L109–L111
- Hanel \latinet al. 1981 Hanel, R. \latinet al. Infrared observations of the Saturnian system from Voyager 1. Science 1981, 212, 192–200
- Marten \latinet al. 1988 Marten, A.; Gautier, D.; Tanguy, L.; Lecacheux, A.; Rosolen, C.; Paubert, G. Abundance of carbon monoxide in the stratosphere of Titan from millimeter heterodyne observations. Icarus 1988, 76, 558–562
- Tanguy \latinet al. 1990 Tanguy, L.; Bézard, B.; Marten, A.; Gautier, D.; Gérard, E.; Paubert, G.; Lecacheux, A. Stratospheric profile of HCN on Titan from millimeter observations. Icarus 1990, 85, 43–57
- Hidayat \latinet al. 1997 Hidayat, T.; Marten, A.; Bézard, B.; Gautier, D.; Owen, T.; Matthews, H.; Paubert, G. Millimeter and Submillimeter Heterodyne Observations of Titan: Retrieval of the Vertical Profile of HCN and the 12C/13C Ratio. Icarus 1997, 126, 170–182
- Pearce \latinet al. 2019 Pearce, B. K. D.; Ayers, P. W.; Pudritz, R. E. A Consistent Reduced Network for HCN Chemistry in Early Earth and Titan Atmospheres: Quantum Calculations of Reaction Rate Coefficients. The Journal of Physical Chemistry A 2019, 123, 1861–1873
- Lee 1980 Lee, L. C. CN(A X ) and CN(B X ) yields from HCN photodissociation. The Journal of Chemical Physics 1980, 72, 6414–6421
- Fukuzawa \latinet al. 1998 Fukuzawa, K.; Osamura, Y.; Schaefer III, H. F. Are neutral-neutral reactions effective for the carbon-chain growth of cyanopolyynes and polyacetylenes in interstellar space? The Astrophysical Journal 1998, 505, 278
- Wilhelm \latinet al. 2009 Wilhelm, M. J.; Nikow, M.; Letendre, L.; Dai, H.-L. Photodissociation of vinyl cyanide at 193 nm: Nascent product distributions of the molecular elimination channels. The Journal of chemical physics 2009, 130, 044307
- Pearce \latinet al. 2020 Pearce, B. K. D.; Molaverdikhani, K.; Pudritz, R. E.; Henning, T.; Hébrard, E. HCN Production in Titan’s Atmosphere: Coupling Quantum Chemistry and Disequilibrium Atmospheric Modeling. The Astrophysical Journal 2020, 901, 110
- Ennis \latinet al. 2020 Ennis, C.; Cable, M. L.; Hodyss, R.; Maynard-Casely, H. E. Mixed Hydrocarbon and Cyanide Ice Compositions for Titan’s Atmospheric Aerosols: A Ternary-Phase Co-crystal Predicted by Density Functional Theory. ACS Earth and Space Chemistry 2020, 4, 1195–1200
- Anderson \latinet al. 2016 Anderson, C. M.; Samuelson, R. E.; Yung, Y. L.; McLain, J. L. Solid-state photochemistry as a formation mechanism for Titan’s stratospheric C4N2 ice clouds. Geophysical Research Letters 2016, 43, 3088–3094
- Anderson \latinet al. 2010 Anderson, C. M.; Samuelson, R. E.; Bjoraker, G. L.; Achterberg, R. K. Particle size and abundance of HC3N ice in Titan’s lower stratosphere at high northern latitudes. Icarus 2010, 207, 914–922
- Oró 1960 Oró, J. Synthesis of adenine from ammonium cyanide. Biochemical and Biophysical Research Communications 1960, 2, 407–412
- Oró and Kamat 1961 Oró, J.; Kamat, S. Amino-acid synthesis from hydrogen cyanide under possible primitive earth conditions. Nature 1961, 190, 442–443
- Oró 1961 Oró, J. Mechanism of synthesis of adenine from hydrogen cyanide under possible primitive Earth conditions. Nature 1961, 191, 1193–1194
- Oró and Kimball 1961 Oró, J.; Kimball, A. Synthesis of purines under possible primitive earth conditions. I. Adenine from hydrogen cyanide. Archives of biochemistry and biophysics 1961, 94, 217–227
- Shapiro 1984 Shapiro, R. The improbability of prebiotic nucleic acid synthesis. Origins of life 1984, 14, 565–570
- Shapiro 1995 Shapiro, R. The prebiotic role of adenine: a critical analysis. Origins of Life and Evolution of the Biosphere 1995, 25, 83–98
- Jung and Choe 2013 Jung, S. H.; Choe, J. C. Mechanisms of prebiotic adenine synthesis from HCN by oligomerization in the gas phase. Astrobiology 2013, 13, 465–475
- He and Smith 2014 He, C.; Smith, M. A. Identification of nitrogenous organic species in Titan aerosols analogs: Implication for prebiotic chemistry on Titan and early Earth. Icarus 2014, 238, 86–92
- Rahm \latinet al. 2016 Rahm, M.; Lunine, J.; Usher, D.; Shalloway, D. Polymorphism and electronic structure of polyimine and its potential significance for prebiotic chemistry on Titan. PNAS; Proceedings of the National Academy of Sciences 2016, 113, 8121–8126
- Jeilani \latinet al. 2016 Jeilani, Y. A.; Williams, P. N.; Walton, S.; Nguyen, M. T. Unified reaction pathways for the prebiotic formation of RNA and DNA nucleobases. Physical Chemistry Chemical Physics 2016, 18, 20177–20188
- Sandstrom and Rahm 2021 Sandstrom, H.; Rahm, M. The beginning of HCN polymerization: Iminoacetonitrile formation and its implications in astrochemical environments. ACS Earth and Space Chemistry 2021, 5, 2152–2159
- Sharma \latinet al. 2022 Sharma, S.; Sandström, H.; Ruiz, F.; Doğan, R.; Rahm, M. Thermodynamics of HCN-derived Polymers: A Quantum Chemical Study. Authorea Preprints 2022,
- Nguyen \latinet al. 2015 Nguyen, T. L.; Baraban, J. H.; Ruscic, B.; Stanton, J. F. On the HCN–HNC energy difference. The Journal of Physical Chemistry A 2015, 119, 10929–10934
- Moreno \latinet al. 2011 Moreno, R.; Lellouch, E.; Lara, L. M.; Courtin, R.; Bockelée-Morvan, D.; Hartogh, P.; Rengel, M.; Biver, N.; Banaszkiewicz, M.; González, A. First detection of hydrogen isocyanide (HNC) in Titan’s atmosphere. Astron. and Astrophys. 2011, 536, L12
- Lee and Rendell 1991 Lee, T. J.; Rendell, A. P. The structure and energetics of the HCN→ HNC transition state. Chemical physics letters 1991, 177, 491–497
- Mendes \latinet al. 2012 Mendes, M. B.; Buhr, H.; Berg, M. H.; Froese, M.; Grieser, M.; Heber, O.; Jordon-Thaden, B.; Krantz, C.; Novotnỳ, O.; Novotny, S., \latinet al. Cold electron reactions producing the energetic isomer of hydrogen cyanide in interstellar clouds. The Astrophysical Journal Letters 2012, 746, L8
- Bezard \latinet al. 1992 Bezard, B.; Marten, A.; Paubert, G. Saturn VI (Titan). IAUC 1992,
- Balucani \latinet al. 2012 Balucani, N.; Skouteris, D.; Leonori, F.; Petrucci, R.; Hamberg, M.; Geppert, W. D.; Casavecchia, P.; Rosi, M. Combined Crossed Beam and Theoretical Studies of the N(2D) + C2H4 Reaction and Implications for Atmospheric Models of Titan. The Journal of Physical Chemistry A 2012, 116, 10467–10479
- Blair and Harrison 1973 Blair, A.; Harrison, A. Bimolecular Reactions of Trapped Ions. VI. Ion–Molecule Reactions Involving CH5+ and C2H5+. Canadian Journal of Chemistry 1973, 51, 1645–1654
- Vigren \latinet al. 2008 Vigren, E.; Kamińska, M.; Hamberg, M.; Zhaunerchyk, V.; Thomas, R. D.; Danielsson, M.; Semaniak, J.; Andersson, P. U.; Larsson, M.; Geppert, W. D. Dissociative recombination of fully deuterated protonated acetonitrile, CD3CND+: product branching fractions, absolute cross section and thermal rate coefficient. Physical Chemistry Chemical Physics 2008, 10, 4014–4019
- Vigren \latinet al. 2012 Vigren, E.; Semaniak, J.; Hamberg, M.; Zhaunerchyk, V.; Kaminska, M.; Thomas, R.; af Ugglas, M.; Larsson, M.; Geppert, W. Dissociative recombination of nitrile ions with implications for Titan’s upper atmosphere. Plan. & Space Sci. 2012, 60, 102–106, Titan Through Time: A Workshop on Titan’s Formation, Evolution and Fate
- Halpern and Tang 1985 Halpern, J. B.; Tang, X. Production of CN () in the photolysis of acetonitrile at 158 nm. Chemical physics letters 1985, 122, 294–299
- Suto and Lee 1985 Suto, M.; Lee, L. Photoabsorption cross section of CH3CN: Photodissociation rates by solar flux and interstellar radiation. Journal of Geophysical Research: Atmospheres 1985, 90, 13037–13040
- Eden \latinet al. 2003 Eden, S.; Limo-Vieira, P.; Kendall, P.; Mason, N. J.; Hoffmann, S. V.; Spyrou, S. M. High resolution photo-absorption studies of acrylonitrile, C2H3 CN, and acetonitrile, CH3CN. The European Physical Journal D - Atomic, Molecular and Optical Physics 2003, 26, 201–210
- Schwell \latinet al. 2008 Schwell, M.; Jochims, H.-W.; Baumgärtel, H.; Leach, S. VUV photophysics of acetonitrile: Fragmentation, fluorescence and ionization in the 7–22 eV region. Chemical Physics 2008, 344, 164–175
- Cable \latinet al. 2020 Cable, M. L.; Vu, T. H.; Malaska, M. J.; Maynard-Casely, H. E.; Choukroun, M.; Hodyss, R. Properties and Behavior of the Acetonitrile–Acetylene Co-Crystal under Titan Surface Conditions. ACS Earth and Space Chemistry 2020, 4, 1375–1385
- Capone \latinet al. 1981 Capone, L. A.; Prasad, S. S.; Huntress, W. T.; Whitten, R. C.; Dubach, J.; Santhanam, K. Formation of organic molecules on Titan. Nature 1981, 293, 45–46
- Seki \latinet al. 1996 Seki, K.; He, M.; Liu, R.; Okabe, H. Photochemistry of Cyanoacetylene at 193.3 nm. The Journal of Physical Chemistry 1996, 100, 5349–5353
- Winnewisser and Walmsley 1978 Winnewisser, G.; Walmsley, C. M. The detection of HC5N and HC7N in IRC +10216. Astron. and Astrophys. 1978, 70, L37–L39
- Broten \latinet al. 1978 Broten, N. W.; Oka, T.; Avery, L. W.; MacLeod, J. M.; Kroto, H. W. The detection of HC9N in interstellar space. The Astrophysical Journal 1978, 223, L105
- Bell \latinet al. 1982 Bell, M. B.; Feldman, P. A.; Kwok, S.; Matthews, H. E. Detection of HC11N in IRC +10∘216. Nature 1982, 295, 389–391
- Petrie and Osamura 2004 Petrie, S.; Osamura, Y. NCCN and NCCCCN Formation in Titan’s Atmosphere: 2. HNC as a Viable Precursor. The Journal of Physical Chemistry A 2004, 108, 3623–3631
- Osamura and Petrie 2004 Osamura, Y.; Petrie, S. NCCN and NCCCCN Formation in Titan’s Atmosphere: 1. Competing Reactions of Precursor HCCN (3A “) with H (2S) and CH3 (2A ‘). The Journal of Physical Chemistry A 2004, 108, 3615–3622
- Zabarnick and Lin 1989 Zabarnick, S.; Lin, M.-C. Kinetics of CN () radical reactions with HCN, BrCN and CH3CN. Chemical Physics 1989, 134, 185–191
- Yang \latinet al. 1992 Yang, D.; Yu, T.; Lin, M.-C.; Melius, C. CN radical reactions with hydrogen cyanide and cyanogen: Comparison of theory and experiment. The Journal of chemical physics 1992, 97, 222–226
- Yang \latinet al. 1992 Yang, D. L.; Yu, T.; Wang, N. S.; Lin, M.-C. Temperature dependence of cyanogen radical reactions with selected alkanes: CN reactivities towards primary, secondary and tertiary CH bonds. Chemical physics 1992, 160, 307–315
- Halpern and Huang 1997 Halpern, J. B.; Huang, Y. Radiative lifetimes, fluorescence quantum yields and photodissociation of the C2N2 (A) and (B) states: evidence for sterically hindered, triplet mediated crossings to the (X) ground state. Chemical Physics 1997, 222, 71–86
- Khanna \latinet al. 1987 Khanna, R.; Perera-Jarmer, M.; Ospina, M. Vibrational infrared and raman spectra of dicyanoacetylene. Spectrochimica Acta Part A: Molecular Spectroscopy 1987, 43, 421–425
- Samuelson \latinet al. 1997 Samuelson, R.; Mayo, L.; Knuckles, M.; Khanna, R. C4N2 ice in Titan’s north polar stratosphere. Planetary and Space Science 1997, 45, 941–948
- Blank \latinet al. 1998 Blank, D. A.; Suits, A. G.; Lee, Y. T.; North, S. W.; Hall, G. E. Photodissociation of acrylonitrile at 193 nm: A photofragment translational spectroscopy study using synchrotron radiation for product photoionization. The Journal of Chemical Physics 1998, 108, 5784–5794
- Prozument \latinet al. 2013 Prozument, K.; Shaver, R. G.; Ciuba, M. A.; Muenter, J. S.; Park, G. B.; Stanton, J. F.; Guo, H.; Wong, B. M.; Perry, D. S.; Field, R. W. A new approach toward transition state spectroscopy. Faraday Discussions 2013, 163, 33
- Petrie \latinet al. 1991 Petrie, S.; Chirnside, T.; Freeman, C.; McEwan, M. The ion/molecule chemistry of CH2CHCN. International journal of mass spectrometry and ion processes 1991, 107, 319–331
- Petrie \latinet al. 1992 Petrie, S.; Freeman, C. G.; McEwan, M. J. The ion–molecule chemistry of acrylonitrile: astrochemical implications. Monthly Notices of the Royal Astronomical Society 1992, 257, 438–444
- Vigren \latinet al. 2009 Vigren, E.; Hamberg, M.; Zhaunerchyk, V.; Kamińska, M.; Thomas, R. D.; Larsson, M.; Millar, T.; Walsh, C.; Geppert, W. D. The dissociative recombination of protonated acrylonitrile, CH2CHCNH+, with implications for the nitrile chemistry in dark molecular clouds and the upper atmosphere of Titan. The Astrophysical Journal 2009, 695, 317
- Stevenson \latinet al. 2015 Stevenson, J. M.; Fouad, W. A.; Shalloway, D.; Usher, D.; Lunine, J.; Chapman, W. G.; Clancy, P. Solvation of nitrogen compounds in Titan’s seas, precipitates, and atmosphere. Icarus 2015, 256, 1–12
- Sandström and Rahm 2020 Sandström, H.; Rahm, M. Can polarity-inverted membranes self-assemble on Titan? Science Advances 2020, 6
- Kanda \latinet al. 1999 Kanda, K.; Nagata, T.; Ibuki, T. Photodissociation of some simple nitriles in the extreme vacuum ultraviolet region. Chemical Physics 1999, 243, 89–96
- Umemoto \latinet al. 1985 Umemoto, H.; Sugiyama, K.; Tsunashima, S.; Sato, S. Collisional deactivation of N (22P) atoms by simple molecules. Bulletin of the Chemical Society of Japan 1985, 58, 3076–3081
- Vigren \latinet al. 2010 Vigren, E.; Hamberg, M.; Zhaunerchyk, V.; Kaminska, M.; Thomas, R. D.; Trippel, S.; Wester, R.; Zhang, M.; Kashperka, I.; af Ugglas, M., \latinet al. Dissociative recombination of protonated propionitrile, CH3CH2CNH+: implications for Titan’s upper atmosphere. The Astrophysical Journal 2010, 722, 847
- Khanna 2005 Khanna, R. Condensed species in Titan’s atmosphere: Identification of crystalline propionitrile (C2H5CN, CH3CH2CN) based on laboratory infrared data. Icarus 2005, 177, 116–121
- Samuelson \latinet al. 2007 Samuelson, R. E.; Smith, M. D.; Achterberg, R. K.; Pearl, J. C. Cassini CIRS update on stratospheric ices at Titan’s winter pole. Icarus 2007, 189, 63–71
- de Kok \latinet al. 2008 de Kok, R.; Irwin, P. G. J.; Teanby, N. A. Condensation in Titan’s stratosphere during polar winter. Icarus 2008, 197, 572–578
- Nna-Mvondo \latinet al. 2019 Nna-Mvondo, D.; Anderson, C.; Samuelson, R. Detailed infrared study of amorphous to crystalline propionitrile ices relevant to observed spectra of Titan’s stratospheric ice clouds. Icarus 2019, 333, 183–198
- Broten \latinet al. 1984 Broten, N. W.; MacLeod, J. M.; Avery, L. W.; Friberg, P.; Hjalmarson, A.; Hoglund, B.; Irvine, W. M. The detection of interstellar methylcyanoacetylene. The Astrophysical Journal 1984, 276, L25
- Carty \latinet al. 2001 Carty, D.; Le Page, V.; Sims, I. R.; Smith, I. W. Low temperature rate coefficients for the reactions of CN and C2H radicals with allene (CH2CCH2) and methyl acetylene (CH3CCH). Chemical Physics Letters 2001, 344, 310–316
- Balucani \latinet al. 2002 Balucani, N.; Asvany, O.; Kaiser, R.-I.; Osamura, Y. Formation of three C4H3N isomers from the reaction of CN (X) with allene, H2CCCH2 (X1A1), and methylacetylene, CH3CCH (X1A1): a combined crossed beam and ab initio study. The Journal of Physical Chemistry A 2002, 106, 4301–4311
- Zhu \latinet al. 2003 Zhu, Z.; Zhang, Z.; Huang, C.; Pei, L.; Chen, C.; Chen, Y. Kinetics of CCN radical reactions with a series of normal alkanes. The Journal of Physical Chemistry A 2003, 107, 10288–10291
- Wang \latinet al. 2006 Wang, J.; Ding, Y.-h.; Sun, C.-c. Cyanomethylidyne: A reactive carbyne radical. ChemPhysChem 2006, 7, 710–722
- McGuire 2018 McGuire, B. A. 2018 Census of Interstellar, Circumstellar, Extragalactic, Protoplanetary Disk, and Exoplanetary Molecules. The Astrophysical Journal Supplement Series 2018, 239, 17
- Marcelino \latinet al. 2021 Marcelino, N.; Tercero, B.; Agúndez, M.; Cernicharo, J. A study of C4H3N isomers in TMC-1: Line by line detection of HCCCH2CN. Astronomy & Astrophysics 2021, 646, L9
- Lovas \latinet al. 2006 Lovas, F. J.; Remijan, A. J.; Hollis, J. M.; Jewell, P. R.; Snyder, L. E. Hyperfine Structure Identification of Interstellar Cyanoallene toward TMC-1. The Astrophysical Journal 2006, 637, L37–L40
- McGuire \latinet al. 2020 McGuire, B. A.; Burkhardt, A. M.; Loomis, R. A.; Shingledecker, C. N.; Lee, K. L. K.; Charnley, S. B.; Cordiner, M. A.; Herbst, E.; Kalenskii, S.; Momjian, E.; Willis, E. R.; Xue, C.; Remijan, A. J.; McCarthy, M. C. Early Science from GOTHAM: Project Overview, Methods, and the Detection of Interstellar Propargyl Cyanide (HCCCH2CN) in TMC-1. The Astrophysical Journal 2020, 900, L10
- Porco \latinet al. 2006 Porco, C. C. \latinet al. Cassini Observes the Active South Pole of Enceladus. Science 2006, 311, 1393–1401
- Dougherty \latinet al. 2006 Dougherty, M. K.; Khurana, K. K.; Neubauer, F. M.; Russell, C. T.; Saur, J.; Leisner, J. S.; Burton, M. E. Identification of a Dynamic Atmosphere at Enceladus with the Cassini Magnetometer. Science 2006, 311, 1406–1409
- Spahn \latinet al. 2006 Spahn, F. \latinet al. Cassini Dust Measurements at Enceladus and Implications for the Origin of the E Ring. Science 2006, 311, 1416–1418
- Coustenis \latinet al. 1998 Coustenis, A.; Salama, A.; Lellouch, E.; Encrenaz, T.; Bjoraker, G. L.; Samuelson, R. E.; de Graauw, T.; Feuchtgruber, H.; Kessler, M. F. Evidence for water vapor in Titan’s atmosphere from ISO/SWS data. Astron. and Astrophys. 1998, 336, L85–L89
- Cottini \latinet al. 2012 Cottini, V.; Nixon, C. A.; Jennings, D. E.; Anderson, C. M.; Gorius, N.; Bjoraker, G. L.; Coustenis, A.; Teanby, N. A.; Achterberg, R. K.; Bézard, B.; de Kok, R.; Lellouch, E.; Irwin, P. G. J.; Flasar, F. M.; Bampasidis, G. Water vapor in Titan’s stratosphere from Cassini CIRS far-infrared spectra. Icarus 2012, 220, 855–862
- Bauduin \latinet al. 2018 Bauduin, S.; Irwin, P.; Lellouch, E.; Cottini, V.; Moreno, R.; Nixon, C.; Teanby, N.; Ansty, T.; Flasar, F. Retrieval of H2O abundance in Titan’s stratosphere: A (re)analysis of CIRS/Cassini and PACS/Herschel observations. Icarus 2018, 311, 288–305
- Mordaunt \latinet al. 1994 Mordaunt, D. H.; Ashfold, M. N. R.; Dixon, R. N. Dissociation dynamics of H2O (D2O) following photoexcitation at the Lyman- wavelength (121.6 nm). The Journal of Chemical Physics 1994, 100, 7360–7375
- Stief \latinet al. 1975 Stief, L. J.; Payne, W. A.; Klemm, R. B. A flasch photolysis-resonance fluorescence study of the formation of O(1D) in the photolysis of water and the reaction of O(1D) with H2, Ar, and He -. The Journal of Chemical Physics 1975, 62, 4000–4008
- Sander \latinet al. 2006 Sander, S.; Friedl, R.; Golden, D.; Kurylo, M.; Moortgat, G.; Keller-Rudek, H.; Wine, P.; Ravishankara, A.; Kolb, C.; Molina, M.; Finlayson-Pitts, B.; Huie, R.; Orkin, V. Chemical kinetics and photochemical data for use in atmospheric studies. Evaluation number 15. JPL TRS 2006,
- Herron 1999 Herron, J. T. Evaluated chemical kinetics data for reactions of N (2D), N (2P), and N2 () in the gas phase. Journal of Physical and Chemical Reference Data 1999, 28, 1453–1483
- McElroy and McConnell 1971 McElroy, M. B.; McConnell, J. C. Atomic carbon in the atmospheres of Mars and Venus. Journal of Geophysical Research 1971, 76, 6674–6690
- Lutz \latinet al. 1983 Lutz, B. L.; de Bergh, C.; Owen, T. Titan - Discovery of carbon monoxide in its atmosphere. Science 1983, 220, 1374
- Noll \latinet al. 1996 Noll, K. S.; Geballe, T. R.; Knacke, R. F.; Pendleton, Y. J. Titan’s 5 m Spectral Window: Carbon Monoxide and the Albedo of the Surface. Icarus 1996, 124, 625–631
- Hidayat \latinet al. 1998 Hidayat, T.; Marten, A.; Bezard, B.; Gautier, D.; Owen, T.; Matthews, H. E.; Paubert, G. Millimeter and Submillimeter Heterodyne Observations of Titan: The Vertical Profile of Carbon Monoxide in Its Stratosphere. Icarus 1998, 133, 109–133
- Gurwell and Muhleman 2000 Gurwell, M. A.; Muhleman, D. O. CO on Titan: More Evidence for a Well-Mixed Vertical Profile. Icarus 2000, 145, 653–656
- Gurwell 2004 Gurwell, M. A. Submillimeter Observations of Titan: Global Measures of Stratospheric Temperature, CO, HCN, HC3N, and the Isotopic Ratios 12C/13C and 14N/15N. Astrophys. J. Lett. 2004, 616, L7–L10
- Glicker and Stief 1971 Glicker, S.; Stief, L. Photolysis of formaldehyde at 1470 and 1236 Å. The Journal of Chemical Physics 1971, 54, 2852–2857
- Cooper \latinet al. 1996 Cooper, G.; Anderson, J. E.; Brion, C. Absolute photoabsorption and photoionization of formaldehyde in the VUV and soft X-ray regions (3–200 eV). Chemical physics 1996, 209, 61–77
- Meller and Moortgat 2000 Meller, R.; Moortgat, G. K. Temperature dependence of the absorption cross sections of formaldehyde between 223 and 323 K in the wavelength range 225–375 nm. Journal of Geophysical Research: Atmospheres 2000, 105, 7089–7101
- Tsang and Hampson 1986 Tsang, W.; Hampson, R. Chemical kinetic data base for combustion chemistry. Part I. Methane and related compounds. Journal of physical and chemical reference data 1986, 15, 1087–1279
- Sander \latinet al. 2006 Sander, S. P.; Abbatt, J.; Barker, J.; Burkholder, J.; Friedl, R.; Golden, D.; Huie, R.; Kolb, C.; Kurylo, M.; Moortgat, G., \latinet al. Chemical kinetics and photochemical data for use in atmospheric studies, Evaluation No. 17. JPL Publication 2006,
- Hörst \latinet al. 2012 Hörst, S.; Yelle, R.; Buch, A.; Carrasco, N.; Cernogora, G.; Dutuit, O.; Quirico, E.; Sciamma-O'Brien, E.; Smith, M.; Somogyi, Á.; Szopa, C.; Thissen, R.; Vuitton, V. Formation of Amino Acids and Nucleotide Bases in a Titan Atmosphere Simulation Experiment. Astrobiology 2012, 12, 809–817
- Samuelson \latinet al. 1983 Samuelson, R. E.; Maguire, W. C.; Hanel, R. A.; Kunde, V. G.; Jennings, D. E.; Yung, Y. L.; Aikin, A. C. CO2 on Titan. J. Geophys. Res. 1983, 88, 8709–8715
- Coustenis and Bezard 1995 Coustenis, A.; Bezard, B. Titan’s atmosphere from Voyager infrared observations. 4: Latitudinal variations of temperature and composition. Icarus 1995, 115, 126–140
- de Kok \latinet al. 2007 de Kok, R.; Irwin, P. G. J.; Teanby, N. A.; Lellouch, E.; Bézard, B.; Vinatier, S.; Nixon, C. A.; Fletcher, L.; Howett, C.; Calcutt, S. B.; Bowles, N. E.; Flasar, F. M.; Taylor, F. W. Oxygen compounds in Titan’s stratosphere as observed by Cassini CIRS. Icarus 2007, 186, 354–363
- Okabe \latinet al. 1978 Okabe, H., \latinet al. Photochemistry of small molecules, 1st ed.; John Wiley and Sons, 1978; Vol. 1
- Chan \latinet al. 1993 Chan, W.; Cooper, G.; Brion, C. The electronic spectrum of carbon dioxide. Discrete and continuum photoabsorption oscillator strengths (6–203 eV). Chemical physics 1993, 178, 401–413
- Yoshino \latinet al. 1996 Yoshino, K.; Esmond, J.; Sun, Y.; Parkinson, W.; Ito, K.; Matsui, T. Absorption cross section measurements of carbon dioxide in the wavelength region 118.7–175.5 nm and the temperature dependence. Journal of Quantitative Spectroscopy and Radiative Transfer 1996, 55, 53–60
- Parkinson \latinet al. 2003 Parkinson, W.; Rufus, J.; Yoshino, K. Absolute absorption cross section measurements of CO2 in the wavelength region 163–200 nm and the temperature dependence. Chemical physics 2003, 290, 251–256
- Stark \latinet al. 2007 Stark, G.; Yoshino, K.; Smith, P.; Ito, K. Photoabsorption cross section measurements of CO2 between 106.1 and 118.7 nm at 295 and 195 K. Journal of Quantitative Spectroscopy and Radiative Transfer 2007, 103, 67–73
- Shemansky 1972 Shemansky, D. CO2 extinction coefficient 1700–3000 Å. The journal of chemical physics 1972, 56, 1582–1587
- Vinatier \latinet al. 2015 Vinatier, S.; Bézard, B.; Lebonnois, S.; Teanby, N. A.; Achterberg, R. K.; Gorius, N.; Mamoutkine, A.; Guandique, E.; Jolly, A.; Jennings, D. E.; Flasar, F. M. Seasonal variations in Titan’s middle atmosphere during the northern spring derived from Cassini/CIRS observations. Icarus 2015, 250, 95–115
- Cabane \latinet al. 1992 Cabane, M.; Chassefière, E.; Israel, G. Formation and growth of photochemical aerosols in Titan’s atmosphere. Icarus 1992, 96, 176–189
- Rannou \latinet al. 1995 Rannou, P.; Cabane, M.; Chassefiere, E.; Botet, R.; McKay, C.; Courtin, R. Titan’s Geometric Albedo: Role of the Fractal Structure of the Aerosols. Icarus 1995, 118, 355–372
- Rannou \latinet al. 2000 Rannou, P.; Ferrari, C.; Rages, K.; Roos-Serote, M.; Cabane, M. Characterization of Aerosols in the Detached Haze Layer of Titan. Icarus 2000, 147, 267–281
- Sagan \latinet al. 1993 Sagan, C.; Khare, B. N.; Thompson, W. R.; McDonald, G. D.; Wing, M. R.; Bada, J. L.; Vo-Dinh, T.; Arakawa, E. T. Polycyclic aromatic hydrocarbons in the atmospheres of Titan and Jupiter. The Astrophysical Journal 1993, 414, 399
- Frenklach and Wang 1991 Frenklach, M.; Wang, H. Detailed modeling of soot particle nucleation and growth. Symposium (International) on Combustion 1991, 23, 1559–1566, Twenty-Third Symposium (International) on Combustion
- Coates 2009 Coates, A. J. Interaction of Titan’s ionosphere with Saturn’s magnetosphere. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 2009, 367, 773–788
- Vuitton \latinet al. 2009 Vuitton, V.; Lavvas, P.; Yelle, R.; Galand, M.; Wellbrock, A.; Lewis, G.; Coates, A.; Wahlund, J.-E. Negative ion chemistry in Titan’s upper atmosphere. Planetary and Space Science 2009, 57, 1558–1572
- Wellbrock \latinet al. 2019 Wellbrock, A.; Coates, A.; Jones, G.; Vuitton, V.; Lavvas, P.; Desai, R.; Waite, J. Heavy negative ion growth in Titan’s polar winter. Monthly Notices of the Royal Astronomical Society 2019, 490, 2254–2261
- Dubois \latinet al. 2019 Dubois, D.; Carrasco, N.; Bourgalais, J.; Vettier, L.; Desai, R. T.; Wellbrock, A.; Coates, A. J. Nitrogen-containing anions and tholin growth in Titan’s ionosphere: implications for Cassini CAPS-ELS observations. The Astrophysical journal letters 2019, 872, L31
- Mukundan and Bhardwaj 2018 Mukundan, V.; Bhardwaj, A. A model for negative ion chemistry in Titan’s ionosphere. The Astrophysical Journal 2018, 856, 168
- Knyazev and Slagle 2001 Knyazev, V. D.; Slagle, I. R. Kinetics of the Reactions of Allyl and Propargyl Radicals with CH3. The Journal of Physical Chemistry A 2001, 105, 3196–3204
- Loison \latinet al. 2006 Loison, J.-C.; Bergeat, A.; Caralp, F.; Hannachi, Y. Rate Constants and H Atom Branching Ratios of the Gas-Phase Reactions of Methylidyne CH () Radical with a Series of Alkanes. The Journal of Physical Chemistry A 2006, 110, 13500–13506
- Hewett \latinet al. 2020 Hewett, D.; Bernath, P. F.; Wong, A.; Billinghurst, B. E.; Zhao, J.; Lombardo, N. A.; Nixon, C. A.; Jennings, D. E. N2 and H2 broadened isobutane infrared absorption cross sections and butane upper limits on Titan. Icarus 2020, 344, 113460
- Cerceau \latinet al. 1985 Cerceau, F.; Raulin, F.; Courtin, R.; Gautier, D. Infrared spectra of gaseous mononitriles: Application to the atmosphere of Titan. Icarus 1985, 62, 207–220
- Coustenis \latinet al. 1993 Coustenis, A.; Encrenaz, T.; Bezard, B.; Bjoraker, G.; Graner, G.; Dang-Nhu, M.; Arie, E. Modeling Titan’s thermal infrared spectrum for high-resolution space observations. Icarus 1993, 102, 240–260
- Vuitton \latinet al. 2006 Vuitton, V.; Yelle, R. V.; Anicich (Retired), V. G. The Nitrogen Chemistry of Titan’s Upper Atmosphere Revealed. The Astrophysical Journal 2006, 647, L175–L178
- Nixon \latinet al. 2010 Nixon, C. A. \latinet al. Upper limits for undetected trace species in the stratosphere of Titan. Faraday Discussions 2010, 147, 65–+
- Teanby \latinet al. 2018 Teanby, N. A.; Cordiner, M. A.; Nixon, C. A.; Irwin, P. G. J.; Hörst, S. M.; Sylvestre, M.; Serigano, J.; Thelen, A. E.; Richards, A. M. S.; Charnley, S. B. The Origin of Titan’s External Oxygen: Further Constraints from ALMA Upper Limits on CS and CH2NH. The Astronomical Journal 2018, 155, 251
- Rinsland \latinet al. 2005 Rinsland, C. P.; Sharpe, S. W.; Sams, R. L. Temperature-dependent infrared absorption cross sections of methyl cyanide (acetonitrile). Journal of Quantitative Spectroscopy and Radiative Transfer 2005, 96, 271–280
- Rinsland \latinet al. 2008 Rinsland, C.; Malathy Devi, V.; Chris Benner, D.; Blake, T.; Sams, R.; Brown, L.; Kleiner, I.; Dehayem-Kamadjeu, A.; Müller, H.; Gamache, R.; Niles, D.; Masiello, T. Multispectrum analysis of the band of CH3CN: Positions, intensities, self- and N2-broadening, and pressure-induced shifts. Journal of Quantitative Spectroscopy and Radiative Transfer 2008, 109, 974–994, Spectroscopy and Radiative Transfer in Planetary Atmospheres
- Wong \latinet al. 2002 Wong, A.-S.; Morgan, C. G.; Yung, Y. L.; Owen, T. Evolution of CO on Titan. Icarus 2002, 155, 382–392
- Seager \latinet al. 2016 Seager, S.; Bains, W.; Petkowski, J. Toward a List of Molecules as Potential Biosignature Gases for the Search for Life on Exoplanets and Applications to Terrestrial Biochemistry. Astrobiology 2016, 16, 465–485
- Khare \latinet al. 1984 Khare, B.; Sagan, C.; Thompson, W.; Arakawa, E.; Suits, F.; Callcott, T.; Williams, M.; Shrader, S.; Ogino, H.; Willingham, T.; Nagy, B. The organic aerosols of Titan. Advances in Space Research 1984, 4, 59 – 68
- Endres \latinet al. 2016 Endres, C. P.; Schlemmer, S.; Schilke, P.; Stutzki, J.; Müller, H. S. The cologne database for molecular spectroscopy, CDMS, in the virtual atomic and molecular data centre, VAMDC. Journal of Molecular Spectroscopy 2016, 327, 95–104
- Delahaye \latinet al. 2021 Delahaye, T.; Armante, R.; Scott, N.; Jacquinet-Husson, N.; Chédin, A.; Crépeau, L.; Crevoisier, C.; Douet, V.; Perrin, A.; Barbe, A., \latinet al. The 2020 edition of the GEISA spectroscopic database. Journal of Molecular Spectroscopy 2021, 380, 111510
- Gordon \latinet al. 2022 Gordon, I.; Rothman, L.; Hargreaves, R.; Hashemi, R.; Karlovets, E.; Skinner, F.; Conway, E.; Hill, C.; Kochanov, R.; Tan, Y., \latinet al. The HITRAN2020 molecular spectroscopic database. Journal of quantitative spectroscopy and radiative transfer 2022, 277, 107949
- Yamamoto \latinet al. 1987 Yamamoto, S.; Saito, S.; Ohishi, M.; Suzuki, H.; Ishikawa, S.-I.; Kaifu, N.; Murakami, A. Laboratory and astronomical detection of the cyclic C3H radical. The Astrophysical Journal 1987, 322, L55
- Cernicharo \latinet al. 2021 Cernicharo, J.; Agúndez, M.; Cabezas, C.; Tercero, B.; Marcelino, N.; Pardo, J. R.; de Vicente, P. Pure hydrocarbon cycles in TMC-1: Discovery of ethynyl cyclopropenylidene, cyclopentadiene, and indene. Astronomy & Astrophysics 2021, 649, L15
- Cernicharo \latinet al. 2021 Cernicharo, J.; Agúndez, M.; Kaiser, R. I.; Cabezas, C.; Tercero, B.; Marcelino, N.; Pardo, J. R.; de Vicente, P. Discovery of benzyne, o-C6H4, in TMC-1 with the QUIJOTE line survey. Astronomy & Astrophysics 2021, 652, L9
- Trainer \latinet al. 2021 Trainer, M.; Brinckerhoff, W.; Grubisic, A.; Danell, R.; Kaplan, D.; van Amerom, F.; Li, X.; Freissinet, C.; Szopa, C.; Buch, A., \latinet al. Development of the Dragonfly Mass Spectrometer (DraMS) for Titan. Lunar and Planetary Science Conference. 2021; p 1532
- SAGAN 1972 SAGAN, C. Interstellar Organic Chemistry. Nature 1972, 238, 77–80
- Donn 1968 Donn, B. Polycyclic Hydrocarbons, Platt Particles, and Interstellar Extinction. The Astrophysical Journal 1968, 152, L129
- Herbig 1995 Herbig, G. H. The Diffuse Interstellar Bands. Annual Review of Astronomy and Astrophysics 1995, 33, 19–73
- McCarthy and McGuire 2021 McCarthy, M. C.; McGuire, B. A. Aromatics and Cyclic Molecules in Molecular Clouds: A New Dimension of Interstellar Organic Chemistry. The Journal of Physical Chemistry A 2021, 125, 3231–3243
- Dinelli \latinet al. 2013 Dinelli, B. M.; López-Puertas, M.; Adriani, A.; Moriconi, M. L.; Funke, B.; García-Comas, M.; D’Aversa, E. An unidentified emission in Titan’s upper atmosphere. Geophys. Res. Lett. 2013, 40, 1489–1493
- Lopez-Puertas \latinet al. 2013 Lopez-Puertas, M.; Funke, B.; Garcia-Comas, M.; Dinelli, B. M.; Adriani, A.; D’Aversa, E.; Moriconi, M. L.; Boersma, C.; Allamandola, E.-m. p., L. J. Large Abundances of Polycyclic Aromatic Hydrocarbons in Titan’s Upper Atmosphere. Astrophysical Journal 2013, 770
- Bauschlicher \latinet al. 2010 Bauschlicher, C. W.; Boersma, C.; Ricca, A.; Mattioda, A. L.; Cami, J.; Peeters, E.; de Armas, F. S.; Saborido, G. P.; Hudgins, D. M.; Allamandola, L. J. The NASA Ames Polycyclic Aromatic Hydrocarbon Infrared Spectroscopic Database: The Computer Spectra. The Astrophysical Journal Supplement Series 2010, 189, 341–351
- Trainer \latinet al. 2013 Trainer, M. G.; Sebree, J. A.; Yoon, Y. H.; Tolbert, M. A. The Influence of Benzene as a Trace Reactant in Titan Aerosol Analogs. The Astrophysical Journal 2013, 766, L4
- Gautier \latinet al. 2017 Gautier, T.; Sebree, J. A.; Li, X.; Pinnick, V. T.; Grubisic, A.; Loeffler, M. J.; Getty, S. A.; Trainer, M. G.; Brinckerhoff, W. B. Influence of trace aromatics on the chemical growth mechanisms of Titan aerosol analogues. Planetary and Space Science 2017, 140, 27–34
- Delitsky and McKay 2010 Delitsky, M.; McKay, C. The photochemical products of benzene in Titan’s upper atmosphere. Icarus 2010, 207, 477–484
- Tomasko \latinet al. 2008 Tomasko, M.; Doose, L.; Engel, S.; Dafoe, L.; West, R.; Lemmon, M.; Karkoschka, E.; See, C. A model of Titan’s aerosols based on measurements made inside the atmosphere. Planetary and Space Science 2008, 56, 669–707, Titan as seen from Huygens - Part 2
- Kroto \latinet al. 1985 Kroto, H. W.; Heath, J. R.; O’Brien, S. C.; Curl, R. F.; Smalley, R. E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163
- Kroto \latinet al. 1991 Kroto, H. W.; Allaf, A.; Balm, S. C60: Buckminsterfullerene. Chemical Reviews 1991, 91, 1213–1235
- Cami \latinet al. 2010 Cami, J.; Bernard-Salas, J.; Peeters, E.; Malek, S. E. Detection of C60 and C70 in a Young Planetary Nebula. Science 2010, 329, 1180–1182
- Cordiner \latinet al. 2019 Cordiner, M. A.; Linnartz, H.; Cox, N. L. J.; Cami, J.; Najarro, F.; Proffitt, C. R.; Lallement, R.; Ehrenfreund, P.; Foing, B. H.; Gull, T. R.; Sarre, P. J.; Charnley, S. B. Confirming Interstellar C Using the Hubble Space Telescope. The Astrophysical Journal 2019, 875, L28
- Becker \latinet al. 1999 Becker, L.; Bunch, T. E.; Allamandola, L. J. Higher fullerenes in the Allende meteorite. Nature 1999, 400, 227–228
- Sittler \latinet al. 2020 Sittler, E. C.; Cooper, J. F.; Sturner, S. J.; Ali, A. Titan’s ionospheric chemistry, fullerenes, oxygen, galactic cosmic rays and the formation of exobiological molecules on and within its surfaces and lakes. Icarus 2020, 344, 113246, Cassini Mission Science Results
- Coy \latinet al. 2023 Coy, B. P.; Nixon, C. A.; Rowe-Gurney, N.; Achterberg, R.; Lombardo, N. A.; Fletcher, L. N.; Irwin, P. Spitzer IRS Observations of Titan as a Precursor to JWST MIRI Observations. The Planetary Science Journal 2023, 4, 114
- Nixon \latinet al. 2016 Nixon, C. A.; Achterberg, R. K.; Ádámkovics, M.; Bézard, B.; Bjoraker, G. L.; Cornet, T.; Hayes, A. G.; Lellouch, E.; Lemmon, M. T.; López-Puertas, M.; Rodriguez, S.; Sotin, C.; Teanby, N. A.; Turtle, E. P.; West, R. A. Titan Science with the James Webb Space Telescope. Publications of the Astronomical Society of the Pacific 2016, 128, 018007
- Charnley \latinet al. 2005 Charnley, S. B.; Kuan, Y.-J.; Huang, H.-C.; Botta, O.; Butner, H. M.; Cox, N.; Despois, D.; Ehrenfreund, P.; Kisiel, Z.; Lee, Y.-Y.; et al., Astronomical searches for nitrogen heterocycles. Advances in Space Research 2005, 36, 137–145
- Sebree \latinet al. 2014 Sebree, J. A.; Trainer, M. G.; Loeffler, M. J.; Anderson, C. M. Titan aerosol analog absorption features produced from aromatics in the far infrared. Icarus 2014, 236, 146–152
- Gautier \latinet al. 2014 Gautier, T.; Carrasco, N.; Schmitz-Afonso, I.; Touboul, D.; Szopa, C.; Buch, A.; Pernot, P. Nitrogen incorporation in Titan’s tholins inferred by high resolution orbitrap mass spectrometry and gas chromatography–mass spectrometry. Earth and Planetary Science Letters 2014, 404, 33–42
- Nixon \latinet al. 2013 Nixon, C. A.; Teanby, N. A.; Irwin, P.; Hörst, S. M. Upper limits for PH3 and H2S in Titan’s atmosphere from Cassini CIRS. Icarus 2013, 224, 253–256
- Sousa-Silva \latinet al. 2020 Sousa-Silva, C.; Seager, S.; Ranjan, S.; Petkowski, J. J.; Zhan, Z.; Hu, R.; Bains, W. Phosphine as a Biosignature Gas in Exoplanet Atmospheres. Astrobiology 2020, 20, 235–268
- Cockell \latinet al. 2021 Cockell, C. S.; McMahon, S.; Biddle, J. F. When is Life a Viable Hypothesis? The Case of Venusian Phosphine. Astrobiology 2021, 21, 261–264
- Hickson \latinet al. 2014 Hickson, K. M.; Loison, J. C.; Cavalié, T.; Hébrard, E.; Dobrijevic, M. The evolution of infalling sulfur species in Titan’s atmosphere. Astronomy & Astrophysics 2014, 572, A58
- Crovisier \latinet al. 2009 Crovisier, J.; Biver, N.; Bockelée-Morvan, D.; Boissier, J.; Colom, P.; Lis, D. C. The chemical diversity of comets: synergies between space exploration and ground-based radio observations. Earth, Moon, and Planets 2009, 105, 267–272
- Waite \latinet al. 2009 Waite, J. H., Jr. \latinet al. Liquid water on Enceladus from observations of ammonia and 40Ar in the plume. Nature 2009, 460, 487–490
- Postberg \latinet al. 2023 Postberg, F.; Sekine, Y.; Klenner, F.; Glein, C. R.; Zou, Z.; Abel, B.; Furuya, K.; Hillier, J. K.; Khawaja, N.; Kempf, S., \latinet al. Detection of phosphates originating from Enceladus’s ocean. Nature 2023, 618, 489–493
- Mumma and Charnley 2011 Mumma, M. J.; Charnley, S. B. The chemical composition of comets—emerging taxonomies and natal heritage. Annual Review of Astronomy and Astrophysics 2011, 49, 471–524
- Smyth and Marconi 2006 Smyth, W. H.; Marconi, M. L. Europa’s atmosphere, gas tori, and magnetospheric implications. Icarus 2006, 181, 510–526
- Bézard \latinet al. 1998 Bézard, B.; Feuchtgruber, H.; Moses, J.; Encrenaz, T. Detection of methyl radicals (CH_3) on Saturn. Astronomy and Astrophysics, v. 334, p. L41-L44 (1998) 1998, 334, L41–L44
- Kunde \latinet al. 2004 Kunde, V. G. \latinet al. Jupiter’s Atmospheric Composition from the Cassini Thermal Infrared Spectroscopy Experiment. Science 2004, 305, 1582–1587
- Ehrenfreund and Charnley 2000 Ehrenfreund, P.; Charnley, S. B. Organic molecules in the interstellar medium, comets, and meteorites: a voyage from dark clouds to the early Earth. Annual Review of Astronomy and Astrophysics 2000, 38, 427–483
- Sandford \latinet al. 2020 Sandford, S. A.; Nuevo, M.; Bera, P. P.; Lee, T. J. Prebiotic astrochemistry and the formation of molecules of astrobiological interest in interstellar clouds and protostellar disks. Chemical reviews 2020, 120, 4616–4659
- Chyba \latinet al. 1990 Chyba, C. F.; Thomas, P. J.; Brookshaw, L.; Sagan, C. Cometary delivery of organic molecules to the early Earth. Science 1990, 249, 366–373
- Chyba and Sagan 1992 Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origins of life. Nature 1992, 355, 125–132
- Lai \latinet al. 2017 Lai, J. C.-Y.; Cordiner, M. A.; Nixon, C. A.; Achterberg, R. K.; Molter, E. M.; Teanby, N. A.; Palmer, M. Y.; Charnley, S. B.; Lindberg, J. E.; Kisiel, Z.; et al., Mapping Vinyl Cyanide and Other Nitriles in Titan’s Atmosphere Using ALMA. The Astronomical Journal 2017, 154, 206
- Molter \latinet al. 2016 Molter, E. M.; Nixon, C. A.; Cordiner, M. A.; Serigano, J.; Irwin, P. G. J.; Teanby, N. A.; Charnley, S. B.; Lindberg, J. E. ALMA Observations of HCN and its Isotopologues on Titan. Astron. J. 2016, 152, 42
- Jolly \latinet al. 2010 Jolly, A.; Fayt, A.; Benilan, Y.; Jacquemart, D.; Nixon, C. A.; Jennings, D. E. The Bending Mode of Diacetylene: From Laboratory Spectroscopy to the Detection of 13C Isotopologues in Titan’s Atmosphere. Astrophys. J. Lett. 2010, 714, 852–859
- Vander Auwera \latinet al. 2014 Vander Auwera, J.; Fayt, A.; Tudorie, M.; Rotger, M.; Boudon, V.; Franco, B.; Mahieu, E. Self-broadening coefficients and improved line intensities for the band of ethylene near 10.5 m, and impact on ethylene retrievals from Jungfraujoch solar spectra. Journal of Quantitative Spectroscopy and Radiative Transfer 2014, 148, 177–185
- Jolly \latinet al. 2015 Jolly, A.; Cottini, V.; Fayt, A.; Manceron, L.; Kwabia-Tchana, F.; Benilan, Y.; Guillemin, J.-C.; Nixon, C.; Irwin, P. Gas phase dicyanoacetylene (C4N2) on Titan: New experimental and theoretical spectroscopy results applied to Cassini CIRS data. Icarus 2015, 248, 340–346
- Sung \latinet al. 2018 Sung, K.; Toon, G. C.; Drouin, B. J.; Mantz, A. W.; Smith, M. A. H. FT-IR measurements of cold propene (C3H6) cross-sections at temperatures between 150 and 299 K. Journal of Quantitative Spectroscopy and Radiative Transfer 2018, 213, 119–132
- Hewett \latinet al. 2019 Hewett, D.; Bernath, P.; Billinghurst, B. Infrared absorption cross sections of isobutane with hydrogen and nitrogen as broadening gases. Journal of Quantitative Spectroscopy and Radiative Transfer 2019, 227, 226–229
- Sung \latinet al. 2020 Sung, K.; Steffens, B.; Toon, G. C.; Nemchick, D. J.; Smith, M. A. H. Pseudoline parameters to represent n-butane (-C4H10) cross-sections measured in the 7–15 m region for the Titan atmosphere. Journal of Quantitative Spectroscopy and Radiative Transfer 2020, 251, 107011
- Bernath and Fernando 2021 Bernath, P.; Fernando, A. M. Infrared absorption cross sections for hot isobutane in the CH stretching region. Journal of Quantitative Spectroscopy and Radiative Transfer 2021, 269, 107644
- Sorensen \latinet al. 2022 Sorensen, J. J.; Bernath, P. F.; Johnson, R. M.; Dodangodage, R.; Cameron, W. D.; LaBelle, K. Absorption cross sections of n-butane, n-pentane, cyclopentane and cyclohexane. Journal of Quantitative Spectroscopy and Radiative Transfer 2022, 290, 108284
- Bernath \latinet al. 2023 Bernath, P. F.; Dodangodage, R.; Zhao, J.; Billinghurst, B. Infrared absorption cross sections for propene broadened by N2 (450–1250 cm-1) and by H2 (2680–3220 cm-1). Journal of Quantitative Spectroscopy and Radiative Transfer 2023, 296, 108462
- Morales \latinet al. 2010 Morales, S. B.; Le Picard, S. D.; Canosa, A.; Sims, I. R. Experimental measurements of low temperature rate coefficients for neutral–neutral reactions of interest for atmospheric chemistry of Titan, Pluto and Triton: Reactions of the CN radical. Faraday discussions 2010, 147, 155–171
- Balucani \latinet al. 2010 Balucani, N.; Leonori, F.; Petrucci, R.; Stazi, M.; Skouteris, D.; Rosi, M.; Casavecchia, P. Formation of nitriles and imines in the atmosphere of Titan: combined crossed-beam and theoretical studies on the reaction dynamics of excited nitrogen atoms N (2D) with ethane. Faraday discussions 2010, 147, 189–216
- Fleury \latinet al. 2014 Fleury, B.; Carrasco, N.; Gautier, T.; Mahjoub, A.; He, J.; Szopa, C.; Hadamcik, E.; Buch, A.; Cernogora, G. Influence of CO on Titan atmospheric reactivity. Icarus 2014, 238, 221–229
- Mancini \latinet al. 2021 Mancini, L.; Vanuzzo, G.; Marchione, D.; Pannacci, G.; Liang, P.; Recio, P.; Rosi, M.; Skouteris, D.; Casavecchia, P.; Balucani, N. The Reaction N(2D) + CH3CCH (Methylacetylene): A Combined Crossed Molecular Beams and Theoretical Investigation and Implications for the Atmosphere of Titan. The Journal of Physical Chemistry A 2021, 125, 8846–8859
- Vanuzzo \latinet al. 2022 Vanuzzo, G.; Marchione, D.; Mancini, L.; Liang, P.; Pannacci, G.; Recio, P.; Tan, Y.; Rosi, M.; Skouteris, D.; Casavecchia, P., \latinet al. The N(2D) + CH2CHCN (Vinyl Cyanide) Reaction: A Combined Crossed Molecular Beam and Theoretical Study and Implications for the Atmosphere of Titan. The Journal of Physical Chemistry A 2022, 126, 6110–6123
- Cable \latinet al. 2021 Cable, M. L.; Runčevski, T.; Maynard-Casely, H. E.; Vu, T. H.; Hodyss, R. Titan in a test tube: organic co-crystals and implications for Titan mineralogy. Accounts of Chemical Research 2021, 54, 3050–3059
- Czaplinski \latinet al. 2023 Czaplinski, E. C.; Vu, T. H.; Cable, M. L.; Choukroun, M.; Malaska, M. J.; Hodyss, R. Experimental Characterization of the Pyridine: Acetylene Co-crystal and Implications for Titan’s Surface. ACS Earth and Space Chemistry 2023, 7, 597–608
- Moore \latinet al. 2010 Moore, M. H.; Ferrante, R. F.; Moore, W. J.; Hudson, R. Infrared spectra and optical constants of nitrile ices relevant to Titan’s atmosphere. The Astrophysical Journal Supplement Series 2010, 191, 96
- Hudson \latinet al. 2014 Hudson, R.; Ferrante, R.; Moore, M. Infrared spectra and optical constants of astronomical ices: I. Amorphous and crystalline acetylene. Icarus 2014, 228, 276–287
- Hudson \latinet al. 2014 Hudson, R.; Gerakines, P.; Moore, M. Infrared spectra and optical constants of astronomical ices: II. Ethane and ethylene. Icarus 2014, 243, 148–157
- Anderson \latinet al. 2018 Anderson, C.; Nna-Mvondo, D.; Samuelson, R.; McLain, J.; Dworkin, J. The SPECTRAL ice chamber: application to Titan’s stratospheric ice clouds. The Astrophysical Journal 2018, 865, 62
- Abplanalp \latinet al. 2019 Abplanalp, M. J.; Góbi, S.; Kaiser, R. I. On the formation and the isomer specific detection of methylacetylene (CH3CCH), propene (CH3CHCH2), cyclopropane (c-C3H6), vinylacetylene (CH2CHCCH), and 1, 3-butadiene (CH2CHCHCH2) from interstellar methane ice analogues. Physical Chemistry Chemical Physics 2019, 21, 5378–5393
- Nna-Mvondo \latinet al. 2019 Nna-Mvondo, D.; Anderson, C.; Samuelson, R. Detailed infrared study of amorphous to crystalline propionitrile ices relevant to observed spectra of Titan’s stratospheric ice clouds. Icarus 2019, 333, 183–198
- Materese \latinet al. 2020 Materese, C. K.; Gerakines, P. A.; Hudson, R. L. Laboratory Studies of Astronomical Ices: Reaction Chemistry and Spectroscopy. Accounts of Chemical Research 2020, 54, 280–290
- Hudson 2020 Hudson, R. L. Preparation, identification, and low-temperature infrared spectra of two elusive crystalline nitrile ices. Icarus 2020, 338, 113548
- Hudson 2022 Hudson, R. L. Infrared spectra of benzene ices: Reexamination and comparison of two recent papers and the literature. Icarus 2022, 384, 115091
- Hudson \latinet al. 2022 Hudson, R. L.; Yarnall, Y. Y.; Gerakines, P. A. Infrared spectral intensities of amine ices, precursors to amino acids. Astrobiology 2022, 22, 452–461
- Gerakines \latinet al. 2022 Gerakines, P. A.; Yarnall, Y. Y.; Hudson, R. L. Direct measurements of infrared intensities of HCN and H2O+ HCN ices for laboratory and observational astrochemistry. Monthly Notices of the Royal Astronomical Society 2022, 509, 3515–3522
- Hudson and Gerakines 2023 Hudson, R. L.; Gerakines, P. A. Influences on Infrared Spectra of Benzene Ices for Titan, Comets, and Beyond: Annealings, Artifacts, and Isosbestic Points. The Planetary Science Journal 2023, 4, 55
- Gautier \latinet al. 2012 Gautier, T.; Carrasco, N.; Mahjoub, A.; Vinatier, S.; Giuliani, A.; Szopa, C.; Anderson, C. M.; Correia, J.-J.; Dumas, P.; Cernogora, G. Mid-and far-infrared absorption spectroscopy of Titan’s aerosols analogues. Icarus 2012, 221, 320–327
- Carrasco \latinet al. 2012 Carrasco, N.; Gautier, T.; Es-Sebbar, E.-T.; Pernot, P.; Cernogora, G. Volatile products controlling Titan’s tholins production. Icarus 2012, 219, 230–240
- Sciamma-O’Brien \latinet al. 2014 Sciamma-O’Brien, E.; Ricketts, C. L.; Salama, F. The Titan Haze Simulation experiment on COSmIC: Probing Titan’s atmospheric chemistry at low temperature. Icarus 2014, 243, 325–336
- Sciamma-O’Brien \latinet al. 2017 Sciamma-O’Brien, E.; Upton, K. T.; Salama, F. The Titan Haze Simulation (THS) experiment on COSmIC. Part II. Ex-situ analysis of aerosols produced at low temperature. Icarus 2017, 289, 214–226
- He \latinet al. 2018 He, C.; Hörst, S. M.; Lewis, N. K.; Yu, X.; Moses, J. I.; Kempton, E. M.-R.; McGuiggan, P.; Morley, C. V.; Valenti, J. A.; Vuitton, V. Laboratory simulations of haze formation in the atmospheres of super-Earths and mini-Neptunes: Particle color and size distribution. The Astrophysical Journal Letters 2018, 856, L3
- Yu \latinet al. 2020 Yu, X.; Hörst, S. M.; He, C.; McGuiggan, P.; Kristiansen, K.; Zhang, X. Surface energy of the Titan aerosol analog “tholin”. The Astrophysical Journal 2020, 905, 88
- Dubois \latinet al. 2020 Dubois, D.; Carrasco, N.; Jovanovic, L.; Vettier, L.; Gautier, T.; Westlake, J. Positive ion chemistry in an N2-CH4 plasma discharge: Key precursors to the growth of Titan tholins. Icarus 2020, 338, 113437
- Nuevo \latinet al. 2022 Nuevo, M.; Sciamma-O’Brien, E.; Sandford, S. A.; Salama, F.; Materese, C. K.; Kilcoyne, A. D. The Titan Haze Simulation (THS) experiment on COSmIC. Part III. XANES study of laboratory analogs of Titan tholins. Icarus 2022, 376, 114841
- He \latinet al. 2022 He, C.; Hörst, S. M.; Radke, M.; Yant, M. Optical Constants of a Titan Haze Analog from 0.4 to 3.5 m Determined Using Vacuum Spectroscopy. The Planetary Science Journal 2022, 3, 25
- Li \latinet al. 2022 Li, J.; Yu, X.; Sciamma-O’Brien, E.; He, C.; Sebree, J. A.; Salama, F.; Hörst, S. M.; Zhang, X. A Cross-laboratory Comparison Study of Titan Haze Analogs: Surface Energy. The Planetary Science Journal 2022, 3, 2
- Bézard \latinet al. 2018 Bézard, B.; Vinatier, S.; Achterberg, R. K. Seasonal radiative modeling of Titan’s stratospheric temperatures at low latitudes. Icarus 2018, 302, 437–450
- Lora \latinet al. 2015 Lora, J. M.; Lunine, J. I.; Russell, J. L. GCM simulations of Titan’s middle and lower atmosphere and comparison to observations. Icarus 2015, 250, 516–528
- Faulk \latinet al. 2017 Faulk, S. P.; Mitchell, J. L.; Moon, S.; Lora, J. M. Regional patterns of extreme precipitation on Titan consistent with observed alluvial fan distribution. Nature Geoscience 2017, 10, 827–831
- Newman \latinet al. 2011 Newman, C. E.; Lee, C.; Lian, Y.; Richardson, M. I.; Toigo, A. D. Stratospheric superrotation in the TitanWRF model. Icarus 2011, 213, 636–654
- Newman \latinet al. 2016 Newman, C. E.; Richardson, M. I.; Lian, Y.; Lee, C. Simulating Titan’s methane cycle with the TitanWRF General Circulation Model. Icarus 2016, 267, 106–134
- Lombardo and Lora 2023 Lombardo, N. A.; Lora, J. M. Influence of observed seasonally varying composition on Titan’s stratospheric circulation. Icarus 2023, 390, 115291
- Dobrijevic \latinet al. 2021 Dobrijevic, M.; Loison, J.; Hue, V.; Cavalié, T. One dimension photochemical models in global mean conditions in question: Application to Titan. Icarus 2021, 364, 114477
- Coustenis \latinet al. 2009 Coustenis, A. \latinet al. TandEM: Titan and Enceladus mission. Experimental Astronomy 2009, 23, 893–946
- Nixon \latinet al. 2016 Nixon, C. A.; Kirchman, F.; Esper, J.; Folta, D.; Mashiku, A. Aerocapture design study for a Titan polar orbiter. 2016 IEEE Aerospace Conference. 2016; pp 1–16
- Rodriguez \latinet al. 2022 Rodriguez, S. \latinet al. Science goals and new mission concepts for future exploration of Titan’s atmosphere, geology and habitability: titan POlar scout/orbitEr and in situ lake lander and DrONe explorer (POSEIDON). Experimental Astronomy 2022,
- Lorenz 2008 Lorenz, R. D. A review of balloon concepts for Titan. Journal of the British Interplanetary Society 2008, 61, 2
- Barnes \latinet al. 2011 Barnes, J. W. \latinet al. AVIATR - Aerial Vehicle for In-situ and Airborne Titan Reconnaissance. Experimental Astronomy 2011, 33, 55–127
- Stofan \latinet al. 2013 Stofan, E.; Lorenz, R.; Lunine, J.; Bierhaus, E. B.; Clark, B.; Mahaffy, P. R.; Ravine, M. TiME-the Titan Mare Explorer. 2013 IEEE Aerospace Conference. 2013; pp 1–10
- Mackenzie \latinet al. 2021 Mackenzie, S. M.; Birch, S. P.; Hörst, S.; Sotin, C.; Barth, E.; Lora, J. M.; Trainer, M. G.; Corlies, P.; Malaska, M. J.; Sciamma-O’Brien, E., \latinet al. Titan: Earth-like on the outside, ocean world on the inside. The Planetary Science Journal 2021, 2, 112
*** For TOC Only ***
