Steady-state Quantum Thermodynamics with Synthetic Negative Temperatures
Abstract
A bath with a negative temperature is a subject of intense debate in recent times. It raises fundamental questions not only on our understanding of negative temperature of a bath in connection with thermodynamics but also on the possibilities of constructing devices using such baths. In this work, we study steady-state quantum thermodynamics involving baths with negative temperatures. A bath with a negative temperature is created synthetically using two baths of positive temperatures and weakly coupling these with a qutrit system. These baths are then coupled to each other via a working system. At steady-state, the laws of thermodynamics are analyzed. We find that whenever the temperatures of these synthetic baths are identical, there is no heat flow, which reaffirms the zeroth law. There is always a spontaneous heat flow for different temperatures. In particular, heat flows from a bath with a negative temperature to a bath with a positive temperature which, in turn, implies that a bath with a negative temperature is ‘hotter’ than a bath with a positive temperature. This warrants an amendment in the Kelvin-Planck statement of the second law, as suggested in earlier studies. In all these processes, the overall entropy production is positive, as required by the Clausius statement of the second law. We construct continuous heat engines operating between positive and negative temperature baths. These engines yield maximum possible heat-to-work conversion efficiency, that is, unity. We also study the thermodynamic nature of heat from a bath with a negative temperature and find that it is thermodynamic work but with negative entropy.
I Introduction
Thermodynamics constitutes a fundamental building block of the modern understanding of nature. With the advent of quantum mechanics, there have been numerous efforts to extend the framework to systems composed of a finite or large number of quantum particles while each particle has a discrete energy spectrum and the states are in a superposition of different energy levels, see for example Binder et al. (2018). One of the possibilities for quantum systems with bounded energy is that, in certain situations, they can assume ‘negative’ temperatures. This arises when the population distribution of a system or bath becomes an inverted Boltzmann distribution, i.e., states with higher energy are populated more than the ones with lower energy. It was first pointed out by Purcell and Pound in the context of nuclear spin systems Purcell and Pound (1951). Subsequently, Ramsey comprehensively discussed the thermodynamic implications of such negative temperatures and the inter-relation between negative and positive temperatures Ramsey (1956). He advocates for an amendment to the Kelvin-Planck statement of the second law to incorporate that heat flows spontaneously from a bath with a negative temperature to one with a positive temperature. In this sense, the negative temperature is ‘hotter’ than a positive temperature.
Initially, Schöpf raised some foundational questions regarding the dynamics of negative temperature Schöpf (1962). He claimed that it is impossible to transform a thermodynamic system adiabatically: from a positive finite temperature to the positive infinite temperature, then from there to a negative infinite (Boltzmann) temperature, and then subsequently to a negative finite (Boltzmann) temperature Schöpf (1962). Tykodi and Tremblay Tykodi (1975); Tremblay (1976); Tykodi (1978) disagreed and showed that the arguments used by Schöpf are thermodynamically inconsistent as these violate the second law of thermodynamics. Nevertheless, the debates on thermodynamics with negative temperatures are not settled. Recent theoretical Mosk (2005); Rapp et al. (2010) and experimental Braun et al. (2013); Carr (2013); Muniz et al. (2023) studies with cold atoms have brought the debate on negative temperatures back again into the spotlight. The works in Dunkel and Hilbert (2013); Hilbert et al. (2014); Hänggi et al. (2016) claim that “all previous negative temperature claims and their implications are invalid as they arise from the use of an entropy definition that is inconsistent both mathematically and thermodynamically." Another study in Struchtrup (2018) states that thermodynamic equilibrium at negative temperatures would be unstable but can be used for work storage or battery. Several researchers have come forward and systematically explained that identification of the thermodynamic entropy exclusively with the volume entropy proposed by Gibbs is the root of all doubts Cerino et al. (2015); Buonsante et al. (2016); Poulter (2016); Abraham and Penrose (2017); Swendsen (2018), and it is inconsistent with the postulates of thermodynamics Wang (2015); Swendsen and Wang (2016). Using Boltzmann entropy as the thermodynamic entropy, they argue that negative temperature is a valid extension of thermodynamics.
Apart from these foundational issues, there are questions on whether a negative temperature bath can be used to construct thermal devices, such as heat engines, refrigerators, heat pumps, etc. Initially, a study on Carnot engines was made by Geusic, et al. Geusic et al. (1967) and, later on, by Landsberg and Nakagomi Landsberg (1977); Nakagomi (1980) in this context. There are also some studies about how the Carnot cycle should be modified in the presence of negative temperatures Dunning-Davies (1976, 1978); Landsberg et al. (1980). Further, some propositions are made to construct a quantum Otto engine Xi and Quan (2017); de Assis et al. (2019) and refrigerators Damas et al. (2022) using a bath with effective negative temperature. It is shown that the heat-to-work conversion efficiency of an engine operating between negative and positive temperatures would be greater than unity Braun et al. (2013); Carr (2013); de Assis et al. (2019). However, much of these models of thermal devices either utilize already existing negative temperature baths without caring how it may be created or effectively prepare one by inverting the populations using some external means.
In this article, we outline how to create a thermal bath with arbitrary temperature, including negative temperature, and study steady-state quantum thermodynamics. The bath is synthesized by letting a quantum system interact simultaneously with two thermal baths at different positive temperatures without external driving. We study various laws of steady-state quantum thermodynamics with these synthetic baths and construct continuous heat engines. We start by proving the zeroth law and show that there is no net heat flow whenever two such baths with identical temperatures are brought in contact with each other. This, in turn, legitimizes the notion of the temperature of a synthetic bath (namely, the synthetic temperature). In the case of two different temperatures, we prove the Kelvin-Planck statement of the second law and demonstrate that there is a spontaneous heat flow from a bath with a negative temperature to one with a positive temperature. This corroborates with the finding of Ramsey Ramsey (1956) - baths with negative temperatures are ‘hotter’ than the ones with positive temperatures. Interestingly, in such cases, the entropy flow is opposite to the direction of heat flow which is again expected for the baths with negative temperatures. We also construct Carnot engines involving synthetic baths and find that engines operating between positive and negative temperature baths can yield unit engine efficiency. This leads us to question the physical meaning of heat flow in the presence of a bath with negative temperatures. With a systematic analysis, we show that the heat associated with a bath with a negative temperature is equivalent to work but with a negative entropy flow.
II Synthetic baths and Negative temperatures
In general, naturally occurring thermal equilibrium results in non-negative temperatures. Only in certain situations, as discussed earlier, can the temperatures be negative. Below, we introduce a method through which a bath can be synthesized. The temperatures of these synthetic baths can assume arbitrary values, including negative ones.
The method utilizes a qutrit system, a hot bath () with inverse temperature , and a cold bath () at inverse temperature . The energy levels of the qutrit are denoted by , , , with the corresponding Hamiltonian . As shown in Fig. 1, the hot (cold) bath weakly interacts with the levels and (levels and ). As convention, we consider , Planck constant , and the Boltzmann constant throughout the article. The levels and are not directly coupled. However, they are indirectly linked through the level . When the couplings between the qutrit and the baths are weak and satisfy the Markov condition, the overall dynamics is expressed in terms of the Lindblad or Lindblad-Gorini-Kossakowski-Sudarshan (LGKS) form of master equation Breuer and Petruccione (2007)
| (1) |
Here represents the density matrix corresponding to a state of the qutrit. The first term on the right-hand side of Eq. (1) takes care of the unitary part of the evolution due to the system Hamiltonian . The second and third terms, with the Lindblad super-operators (LSOs) and , represent the contributions due to the dissipative part of the evolution induced by the hot and cold thermal baths, respectively. The LSOs are expressed (for ) as
| (2) |
where , , anti-commutator , and . The coefficient is the Weiskopf-Wigner decay constant. The overall dynamics leads to heat exchange between the baths and the system. The heat fluxes are quantified as due to interaction with the bath Boukobza and Tannor (2006a, b, 2007). Heat flux implies that heat is flowing into the qutrit system from the bath with inverse temperature .
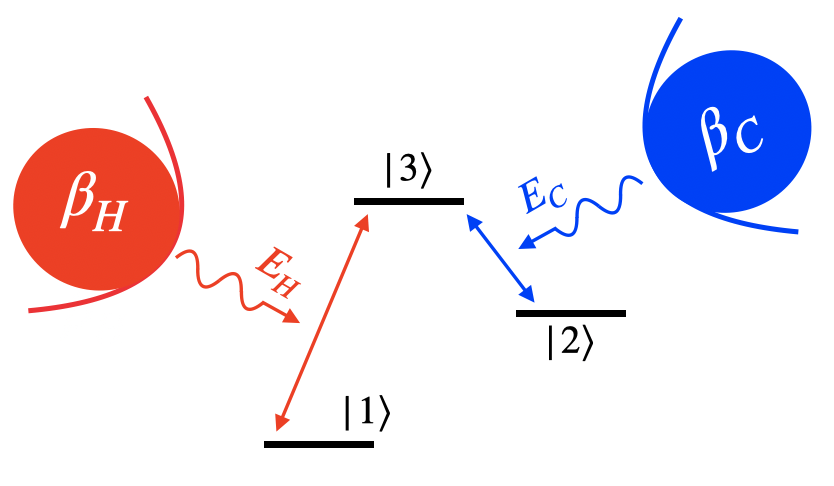
This dynamics always leads to a steady state, say , which is diagonal in the energy eigenstates. The the populations of the states satisfy
The populations corresponding to states and attain thermal equilibrium with the hot bath, and similarly, the populations of and attain thermal equilibrium with the cold bath. In fact, the dynamics drives the overall system to reach thermal equilibrium, albeit in interactions with two baths at different temperatures. This is justified because the heat flux and entropy production vanish, i.e., for and respectively. As the entire system is in thermodynamic equilibrium, so are the populations of the states and .
In general, if one introduces an interaction between the levels and , be it time-dependent or time-independent, the heat and entropy fluxes become non-zero Boukobza and Tannor (2006b, 2007) and the corresponding populations change. But once the interaction is switched off, the populations revert to their equilibrium values. This is as if the levels and , or the subspace spanned by these two levels, are interacting with a synthetic bath at inverse temperature , defined as
| (3) |
where . We note that a Lindblad super-operator (LSO) cannot be given exclusively for the equilibration dynamics due to the synthetic bath. However, as we discuss in the later sections, this is a legitimate thermal bath. We call the ’synthetic’ inverse temperature because it can be tuned to assume arbitrary values, including negative values, by changing the energy-level spacings and the and . In literature, there are debates on whether the temperature of a system can be continuously changed from a positive to a negative equilibrium temperature Struchtrup (2018). However, in this setup, the inverse temperature of the synthetic bath can be tuned continuously, e.g., from to , including (infinite temperature).
III Thermodynamics with synthetic baths
To study thermodynamics with synthetic temperatures, we consider two different qutrit systems and with the corresponding Hamiltonians , with . We assume for both systems, i.e., the energy spacing between and for both and are same. For brevity, we denote and . Each system couples a hot and a cold bath with inverse temperatures and respectively (see Fig. 2) and reaches an equilibrium state. Without an interaction in between, the equilibrium state of the composite becomes
| (4) |
where , , , and . The population ratio between the degenerate energy states and is
| (5) |
where and are the synthetic temperatures corresponding to qutrit and respectively.

An interaction is introduced that only couples subspace spanned by the energy levels belonging to and in each qutrit, ensuring an energy exchange between and only through these subspaces. The most general interaction Hamiltonian that drives an energy exchange between these subspaces is given by
| (6) |
where . This interaction also strictly conserves energy, as . The overall dynamics of is expressed as
| (7) |
where , , and . Here, and are the LSOs taking into account the dissipative part of the dynamics due to the coupling with hot and cold baths, respectively, with the qutrit . Under this dynamics, the composite system reaches a steady state, say . Then, the heat flux and the entropy flux, respectively, are
| (8) |
with , and .
In absence of any interaction between the and , the steady (or equilibrium) state is (see Eq. (4)). Then, the heat flux from is , as . However in presence of interaction via , the steady state becomes , and then the . This means that there is heat flux through the subspace spanned by of , which we may consider as the heat flux due to the synthetic bath associated with . By convention, implies a heat flux from the synthetic bath to , which is then passed to . With these tools at hand, we now set out to explore steady-state quantum thermodynamics with synthetic temperatures. Note that the first law is always respected at steady state as . Thus, our emphasis would be on studying the zeroth and second laws.


Zeroth law - In thermodynamics, the zeroth law interlinks the notion of temperature with equilibrium. It states that if two systems are in thermal equilibrium, they must have the same temperature and vice versa. Again, thermal equilibrium implies that when two systems are in contact, there is no net flux in any thermodynamic quantities (such as heat and entropy) between systems. In such situations, the overall entropy production also vanishes. Below we show that, in the two-qutrit scenario discussed above, there is no net flux of any thermodynamic quantity whenever the synthetic temperatures are identical for both qutrits.
Recall the setup we consider in Fig. 2. Without any interaction, the steady state of is , which is diagonal in the energy eigenstates (see Eq. (4)). With same synthetic inverse temperatures , the populations of the states and satisfy (see Eq. (5)). It means, the matrix corresponding to the state in the subspace spanned by is proportional to an identity operator. Now an interaction between and introduced by , as in Eq. (6). It is easily seen that for . Thus, even after the interaction is switched on, the steady state remains unaltered, i.e., and . Hence, there is no exchange of heat and entropy between and , as for . This implies that the synthetic baths are in thermal equilibrium whenever the synthetic temperatures are identical, irrespective of whether the temperatures are positive or negative. A numerical analysis also confirms this. See Fig. 3(a).
Second law - For , heat and entropy flow is possible from one qutrit to the other. However, the flow cannot be arbitrary. The second law dictates the physically allowed processes given that zeroth and first laws are respected. There are various statements of the second law. Below, we analyze the Kelvin-Planck statement to deal with the directionality of heat flow and the Clausius statement regarding entropy production.
Let us consider the case for which . When there is no interaction between the qutrits, the equilibrium state of the composite is . The state is expressed in the block-diagonal form as
where and . The populations corresponding to the energy eigenstates and satisfy (see Eq. (5)).
Now let us disconnect the thermal baths and introduce an interaction driven by . This, or the total Hamiltonian , evolves the composite and induces a rotation onto the subspace spanned by and only. At the same time, the other part of the density matrix remains unchanged. As a result, there appear off-diagonal elements in this subspace. Say, the state of after any evolution becomes
The unitary nature of the evolution in the subspace and guarantees that and . For this reason and as the off-diagonal elements do not contribute to the populations of the reduced state of , i.e., , we find and . Similarly, for the reduced state of , the modified populations becomes and . Note, the populations corresponding to level for both and remain unchanged, i.e., and . Clearly, the qutrit loses some energy. As the evolution respects strict energy conservation, the qutrit gains the same amount of energy. Thus, any evolution due to ensures that there is an energy flow from to for . After this modification, if is now exposed to its baths, the dissipative dynamics due to forces the qutrit to restore its equilibrium state, and . This, in turn, increases the energy of by absorbing some heat from the hot and cold baths or, equivalently, from the synthetic bath. Similarly, if is exposed to its baths, some of its energy is released to its synthetic bath in the form of heat and thereby attains its equilibrium. Note in this process, to reach the equilibrium, the populations of do not remain constant throughout in both and .
From the above arguments, we see that the unitary evolution with the interaction between and drives the composite out of equilibrium leading to a spontaneous heat flow from to for . While, the dissipative evolution due to thermal interactions with the baths tries to restore the composite back to the initial equilibrium state () by pumping some heat into and absorbing some heat from . When both unitary and dissipative evolutions occur simultaneously, as in Eq. (7), the opposing tendencies balance each other and result in a steady state, say . This steady state is again block-diagonal in total energy eigenstates and has off-diagonal elements in the eigenstates and . Nevertheless, the steady-state dynamics generate a heat flux from to ,
| (9) |
and . The expression of can be given analytically, and it has complicated dependencies with all the parameters. Rather, a numerical analysis is more illuminating; we have done so in Fig. 3(a). Again for , we find that . This means that there is a spontaneous heat flow from to .
In thermodynamics, the Kelvin-Planck statement of the second law states that heat can only flow from a hot bath to a cold bath when no external work is performed. As we see above for , there is a heat flow from to . This means that: (1) baths with negative inverse temperatures are ’hotter’ than the baths with positive inverse temperatures; (2) baths with larger negative inverse temperatures are ’hotter’ than those with smaller negative inverse temperatures. This is in conformation with the findings of Ramsey Ramsey (1956). However, one important point to be noted here is that although there is a heat flow from a negative to a positive temperature bath, the entropy flow is the opposite. This is indeed a signature of a bath having a negative temperature.
The Clausius statement, another formulation of the second law, states that the overall entropy production is always positive in a thermodynamical process. For steady-state thermodynamics with synthetic baths, the overall entropy production rate is given by
| (10) |
where is the rate of change in von Neumann entropy , and () is the synthetic inverse temperature of () and () is the heat flux from (). Note at steady state, the and , as . For , the heat flux from is positive, . Consequently, . Similarly, for , and thus . For , we have . Thus, the Clausius inequality in the differential form is
and it is always satisfied as long as the Kelvin-Planck statement is respected.
At steady state, the entropy production rate is positive, . This is mainly due to the dissipative interaction between the baths and the system. However, one may find out an entropy flow through the system , as
for (see Appendix A). At steady state, as the state does not evolve over time. implies that there is an entropy flux from bath with inverse temperature to via , and similarly for . In general, for a bath with positive temperature, an outflow of heat is associated to a decrease in entropy. One striking feature we must note here is that, although there is a spontaneous heat flow from a bath with negative temperature to a bath with positive temperature, the entropy flow is just opposite to that (see Fig. 3(b)). This is also true when both baths are of negative temperatures. For a bath with negative temperature, an outflow of heat is associated with an increase in entropy of the bath. Thus, a bath with negative temperature in general acts as an entropy sink.
IV Quantum heat engines with a bath at negative temperature
Now we discuss heat engines operating with a bath at synthetic temperatures, particularly at negative temperatures. A device acting as a heat engine aims to transform heat into work. A generic heat engine consists of three primary parts: two separate heat baths with different temperatures and a working system. It operates by absorbing heat from the hot bath. The working system transforms part of this heat into work, dumping the rest into the cold bath. The model of a quantum heat engine (QHE) we are concerned with utilizes a synthetic bath with negative temperature and a heat bath with positive temperature (as depicted in Fig. 4). The working system is composed of a qutrit () and a qubit (). The synthetic bath with inverse temperature is created using two baths at different temperatures and and letting these weakly interact with , similar to the one considered in Fig. 1. The Hamiltonian of is given by . The qubit is weakly coupled to a bath at inverse temperature , and its Hamiltonian is , where . To operate the device as a heat engine, a time-dependent interaction is introduced between and driven by the interaction Hamiltonian
| (11) |
The total Hamiltonian is then , where . The overall dynamics will never lead to a steady state for a time-dependent interaction. However, for a periodic time dependence, there is a rotating frame in which the interaction becomes time-independent. For instance, to move from the laboratory frame to a rotating frame to we may introduce a rotation, given by the unitary which satisfies . For a suitable , the interaction becomes time-independent, i.e., . In this rotating frame, the overall dynamics comprising the unitary and the dissipative evolution is given by (see Appendix A)
| (12) |
for a state , with and . Note the LSOs remain unchanged in the rotating frame. Now that the time-dependence in the Hamiltonian is lifted, the dynamics attains a steady state in the rotating frame.

With this, the heat flux, entropy flux, and the power in the laboratory frame are quantified as (see Appendix A)
| and | (13) |
for , and . Here and represent the heat and entropy fluxes through system respectively, and represents the power. The condition is always satisfied as required by the first law Alicki (1979); Kosloff and Levy (2014) and at steady state, . For any negative inverse temperature , we have, as confirmed by numerical analysis, , , and . This means the device draws heat from both the synthetic bath and the bath with inverse temperature . For traditional engines, the efficiency is calculated as the ratio of work extracted and the heat absorbed by the engine from the hot bath. Here, the heat is absorbed from both baths. For each bath, the corresponding engine efficiency may be defined as
It can be easily checked that and . Thus, the efficiency exceeds unity for an engine operating between baths with positive and negative temperatures. This is what is also claimed in literature Geusic et al. (1967). We, however, find this conclusion incomplete. The heat-to-work conversion efficiency should always be defined with respect to the total amount of heat entering the engine and the amount of work produced out of that. In that sense, the total heat flux entering the engine is , and this entire heat is converted into work. As a result, we find
i.e., the engine efficiency becomes unity. Thus, the efficiency of an engine can never exceed unity in any circumstance as long as the first law, i.e., the overall energy conservation, is respected.

This is, nevertheless, different from what we see in traditional heat engines. It raises a question on the physical meaning of the heat released or absorbed by a bath with a negative temperature. By definition, heat is a form of energy that is always associated with a change in the entropy of the corresponding bath. Heat flow, thus, occurs with an entropy flow. The work, on the other hand, is a pure form of energy and is not associated with any flow of entropy. In traditional engines operating with baths at positive temperatures, the direction of heat flow and the direction of entropy flow is the same. That is how a bath gets cooled down when it releases some heat. For a bath with a negative temperature, this is not true (see Fig. 3(b)). There the bath’s entropy increases as it releases heat. For the engine we have considered above, the direction of heat flux is opposite to the direction of entropy flux in which is coupled to the negative bath (see Fig. 5). While for , coupled to positive temperature bath, the direction of heat flux is the same as the direction of entropy flux . In fact, while heat is entering the engine from both baths, there is an entropy flow from the bath with inverse temperature to the bath with where the latter acts as an entropy sink.
In order to understand the thermodynamic nature of this heat, let us make a closer inspection of the process that is happening on and alone in the rotating frame. To create a synthetic bath with negative temperature, interacts with hot and cold baths with inverse temperatures and . The corresponding heat fluxes are and . Recall that . We can also quantify the power as which is produced in . For the interaction , we always find , , and . For , the heat flux and power can be similarly calculated, and they are and . At steady state, we have
Note and . This may imply that the heat flux from the synthetic bath is quantitatively equal to the power extracted from . But, as mentioned above, this cannot be just power, as power is not associated with any entropy flux. For justification, we may remove the interaction between qutrit and qubit and drive with an oscillating external field instead. This is exactly what is considered in Boukobza and Tannor (2007), where power is quantified as the flux of pure energy being stored in the external field and is not associated with entropy flow (see Appendix B). Here, on the contrary, we see an entropy flux opposite to the power extracted (heat flux) in . This leads us to conclude that heat from a bath with a negative temperature is thermodynamic work but with negative entropy.
V Conclusion
In this article, we have studied steady-state quantum thermodynamics with negative temperatures. For that, we have engineered synthetic baths by utilizing two baths at different positive temperatures and letting them weakly interact with qutrit systems in a particular fashion. These synthetic baths can assume arbitrary temperatures, including negative ones. These baths with negative temperatures are exploited to study steady-state thermodynamics. We have explored the thermodynamic laws, particularly the zeroth and second laws. We have shown that whenever two synthetic baths with identical temperatures are brought in contact, there is no heat flow in between. This, in turn, legitimizes the notion of temperatures in synthetic baths. On the other hand, for non-identical temperatures, there is a heat flow. Further, heat always flows spontaneously from a bath with a negative temperature to a positive one. This implies that a bath with a negative temperature is ‘hotter’ than a bath with a positive temperature. Further, there is a heat flow from a bath with a less negative temperature to a bath with a more negative temperature. We have then studied the Clausius statement of the second law in case of negative temperatures and found that there is always non-negative entropy production in the steady-state thermal processes. Then, we have introduced a heat engine model that operates between two baths, one with negative temperature and the other with positive temperature. Unlike traditional engines, these engines always yield unit heat-to-work conversion efficiency. A systematic analysis has revealed that the heat flow from a bath with a negative temperature is equivalent to an injection of work into the working system by an equal amount. This is exactly the reason why these engines yield unit efficiency.
The proposed engines utilizes qutrit and qubit systems and two or three baths at different temperatures. Thus, these engines can be realized experimentally, for example, using atom-optical Rossnagel et al. (2016); Klatzow et al. (2019); Wang et al. (2022) and NMR Peterson et al. (2019) systems. Finally, we conclude that:
-
•
A thermal bath with a negative temperature can be synthesized with two baths with different positive temperatures.
-
•
In steady-state thermodynamics with negative temperatures, the zeroth law and the Clausius statement of the second law remain unchanged. However, the Kelvin-Plack statement is to be appended to incorporate that there is a spontaneous heat flow from a bath with a negative temperature to a bath with a positive temperature and from a bath with smaller negative temperature to a bath with a larger negative temperature.
-
•
A continuous heat engine can be constructed using baths with negative and positive temperatures. In such engines, the heat-to-work conversion efficiency is always unity. This is maximum for any device that respects first law, i.e., conservation of total energy.
-
•
On the fundamental level, the thermodynamic nature of heat from a bath with a positive temperature is qualitatively different from the one with a negative temperature. For the former, heat flows in the same direction as entropy flow. For the latter, heat flows in the opposite direction of entropy flow. Further, heat from a bath with a negative temperature is thermodynamic work but with negative entropy.
Acknowledgements.
M.L.B., U.B., and M.L. thankfully acknowledge support from: ERC AdG NOQIA; Ministerio de Ciencia y Innovation Agencia Estatal de Investigaciones (PGC2018-097027-B-I00/10.13039/501100011033, CEX2019-000910-S/10.13039/501100011033, Plan National FIDEUA PID2019-106901GB-I00, FPI, QUANTERA MAQS PCI2019-111828-2, QUANTERA DYNAMITE PCI2022-132919, Proyectos de I+D+I “Retos Colaboración" QUSPIN RTC2019-007196-7); MICIIN with funding from European Union NextGenerationEU(PRTR-C17.I1) and by Generalitat de Catalunya; Fundació Cellex; Fundació Mir-Puig; Generalitat de Catalunya (European Social Fund FEDER and CERCA program, AGAUR Grant No. 2021 SGR 01452, QuantumCAT U16-011424, co-funded by ERDF Operational Program of Catalonia 2014-2020); Barcelona Supercomputing Center MareNostrum (FI-2022-1-0042); EU (PASQuanS2.1, 101113690); EU Horizon 2020 FET-OPEN OPTOlogic (Grant No 899794); EU Horizon Europe Program (Grant Agreement 101080086 - NeQST), National Science Centre, Poland (Symfonia Grant No. 2016/20/W/ST4/00314); ICFO Internal “QuantumGaud" project; European Union’s Horizon 2020 research and innovation program under the Marie-Sklodowska-Curie grant agreement No 101029393 (STREDCH) and No 847648 (“La Caixa" Junior Leaders fellowships ID100010434:LCF/BQ/PI19/ 11690013,LCF/ BQ/PI20/ 11760031, LCF/BQ/PR20/11770012,LCF/BQ/ PR21/11840013). M.L.B acknowledges the financial support from MCIN/AEI/10.13039/5011000 11033. V.S. acknowledges the financial support from the Institute for Basic Science (IBS) in the Republic of Korea through the project IBS-R024-D1. M.N.B. gratefully acknowledges financial support from SERB-DST (CRG/2019/002199), Government of India.References
- Binder et al. (2018) Felix Binder, Luis A. Correa, Christian Gogolin, Janet Anders, and Gerardo Adesso, Thermodynamics in the Quantum Regime, Vol. 195 (Springer International Publishing, 2018).
- Purcell and Pound (1951) E. M. Purcell and R. V. Pound, “A nuclear spin system at negative temperature,” Phys. Rev. 81, 279–280 (1951).
- Ramsey (1956) Norman F. Ramsey, “Thermodynamics and statistical mechanics at negative absolute temperatures,” Phys. Rev. 103, 20–28 (1956).
- Schöpf (1962) Hans-Georg Schöpf, “Zur thermodynamik negativer absoluter temperaturen,” Annalen der Physik 464, 107–123 (1962).
- Tykodi (1975) R. J. Tykodi, “Negative kelvin temperatures: Some anomalies and a speculation,” American Journal of Physics 43, 271–273 (1975).
- Tremblay (1976) André-Marie Tremblay, “Comment on ”negative kelvin temperatures: Some anomalies and a speculation”,” American Journal of Physics 44, 994–995 (1976).
- Tykodi (1978) R. J. Tykodi, “Quasi-carnot cycles, negative kelvin temperatures, and the laws of thermodynamics,” American Journal of Physics 46, 354–359 (1978).
- Mosk (2005) A. P. Mosk, “Atomic gases at negative kinetic temperature,” Phys. Rev. Lett. 95, 040403 (2005).
- Rapp et al. (2010) Akos Rapp, Stephan Mandt, and Achim Rosch, “Equilibration rates and negative absolute temperatures for ultracold atoms in optical lattices,” Physical Review Letters 105 (2010).
- Braun et al. (2013) S. Braun, J. P. Ronzheimer, M. Schreiber, S. S. Hodgman, T. Rom, I. Bloch, and U. Schneider, “Negative absolute temperature for motional degrees of freedom,” Science 339, 52–55 (2013).
- Carr (2013) Lincoln D. Carr, “Negative temperatures?” Science 339, 42–43 (2013).
- Muniz et al. (2023) A. L. Marques Muniz, F. O. Wu, P. S. Jung, M. Khajavikhan, D. N. Christodoulides, and U. Peschel, “Observation of photon-photon thermodynamic processes under negative optical temperature conditions,” Science 379, 1019–1023 (2023).
- Dunkel and Hilbert (2013) Jörn Dunkel and Stefan Hilbert, “Consistent thermostatistics forbids negative absolute temperatures,” Nature Physics 10, 67–72 (2013).
- Hilbert et al. (2014) Stefan Hilbert, Peter Hänggi, and Jörn Dunkel, “Thermodynamic laws in isolated systems,” Physical Review E 90 (2014).
- Hänggi et al. (2016) Peter Hänggi, Stefan Hilbert, and Jörn Dunkel, “Meaning of temperature in different thermostatistical ensembles,” Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 374, 20150039 (2016).
- Struchtrup (2018) Henning Struchtrup, “Work storage in states of apparent negative thermodynamic temperature,” Physical Review Letters 120, 250602 (2018).
- Cerino et al. (2015) Luca Cerino, Andrea Puglisi, and Angelo Vulpiani, “A consistent description of fluctuations requires negative temperatures,” Journal of Statistical Mechanics: Theory and Experiment 2015, P12002 (2015).
- Buonsante et al. (2016) Pierfrancesco Buonsante, Roberto Franzosi, and Augusto Smerzi, “On the dispute between boltzmann and gibbs entropy,” Annals of Physics 375, 414–434 (2016).
- Poulter (2016) J. Poulter, “In defense of negative temperature,” Physical Review E 93 (2016), 10.1103/physreve.93.032149.
- Abraham and Penrose (2017) Eitan Abraham and Oliver Penrose, “Physics of negative absolute temperatures,” Physical Review E 95 (2017).
- Swendsen (2018) Robert H Swendsen, “Thermodynamics of finite systems: a key issues review,” Reports on Progress in Physics 81, 072001 (2018).
- Wang (2015) Jian-Sheng Wang, “Critique of the gibbs volume entropy and its implication,” (2015).
- Swendsen and Wang (2016) Robert H. Swendsen and Jian-Sheng Wang, “Negative temperatures and the definition of entropy,” Physica A: Statistical Mechanics and its Applications 453, 24–34 (2016).
- Geusic et al. (1967) J. E. Geusic, E. O. Schulz-DuBios, and H. E. D. Scovil, “Quantum equivalent of the carnot cycle,” Phys. Rev. 156, 343–351 (1967).
- Landsberg (1977) P T Landsberg, “Heat engines and heat pumps at positive and negative absolute temperatures,” Journal of Physics A: Mathematical and General 10, 1773–1770 (1977).
- Nakagomi (1980) T Nakagomi, “Mathematical formulation of the heat-engine theory of thermodynamics including negative absolute temperatures,” Journal of Physics A: Mathematical and General 13, 291–299 (1980).
- Dunning-Davies (1976) J Dunning-Davies, “Negative absolute temperatures and carnot cycles,” Journal of Physics A: Mathematical and General 9, 605–609 (1976).
- Dunning-Davies (1978) J. Dunning-Davies, “Remarks on negative absolute temperatures and carnot cycles,” American Journal of Physics 46, 583–585 (1978).
- Landsberg et al. (1980) P T Landsberg, R J Tykodi, and A M Tremblay, “Systematics of carnot cycles at positive and negative kelvin temperatures,” Journal of Physics A: Mathematical and General 13, 1063–1074 (1980).
- Xi and Quan (2017) Jing-Yi Xi and Hai-Tao Quan, “Quantum heat engine and negative boltzmann temperature,” Communications in Theoretical Physics 68, 347 (2017).
- de Assis et al. (2019) Rogério J. de Assis, Taysa M. de Mendonça, Celso J. Villas-Boas, Alexandre M. de Souza, Roberto S. Sarthour, Ivan S. Oliveira, and Norton G. de Almeida, “Efficiency of a quantum otto heat engine operating under a reservoir at effective negative temperatures,” Physical Review Letters 122 (2019), 10.1103/physrevlett.122.240602.
- Damas et al. (2022) Gabriella G Damas, Rogério J de Assis, and Norton G de Almeida, “Negative temperature is cool for cooling,” arXiv preprint arXiv:2204.09479 (2022).
- Breuer and Petruccione (2007) Heinz-Peter Breuer and Francesco Petruccione, The Theory of Open Quantum Systems (Oxford University PressOxford, 2007).
- Boukobza and Tannor (2006a) E. Boukobza and D. J. Tannor, “Thermodynamics of bipartite systems: Application to light-matter interactions,” Phys. Rev. A 74, 063823 (2006a).
- Boukobza and Tannor (2006b) E. Boukobza and D. J. Tannor, “Thermodynamic analysis of quantum light amplification,” Phys. Rev. A 74, 063822 (2006b).
- Boukobza and Tannor (2007) E. Boukobza and D. J. Tannor, “Three-level systems as amplifiers and attenuators: A thermodynamic analysis,” Phys. Rev. Lett. 98, 240601 (2007).
- Alicki (1979) R Alicki, “The quantum open system as a model of the heat engine,” Journal of Physics A: Mathematical and General 12, L103 (1979).
- Kosloff and Levy (2014) Ronnie Kosloff and Amikam Levy, “Quantum heat engines and refrigerators: Continuous devices,” Annual Review of Physical Chemistry 65, 365–393 (2014).
- Rossnagel et al. (2016) Johannes Rossnagel, Samuel T. Dawkins, Karl N. Tolazzi, Obinna Abah, Eric Lutz, Ferdinand Schmidt-Kaler, and Kilian Singer, “A single-atom heat engine,” Science 352, 325–329 (2016).
- Klatzow et al. (2019) James Klatzow, Jonas N. Becker, Patrick M. Ledingham, Christian Weinzetl, Krzysztof T. Kaczmarek, Dylan J. Saunders, Joshua Nunn, Ian A. Walmsley, Raam Uzdin, and Eilon Poem, “Experimental demonstration of quantum effects in the operation of microscopic heat engines,” Phys. Rev. Lett. 122, 110601 (2019).
- Wang et al. (2022) Kunkun Wang, Ruqiao Xia, Léa Bresque, and Peng Xue, “Experimental demonstration of a quantum engine driven by entanglement and local measurements,” Phys. Rev. Res. 4, L032042 (2022).
- Peterson et al. (2019) John P. S. Peterson, Tiago B. Batalhão, Marcela Herrera, Alexandre M. Souza, Roberto S. Sarthour, Ivan S. Oliveira, and Roberto M. Serra, “Experimental characterization of a spin quantum heat engine,” Phys. Rev. Lett. 123, 240601 (2019).
Appendix A Rotating frame and steady-state thermodynamics
Let us consider the setup discussed in Section IV of the main text. The Hamiltonian of the working systems are
where . The Hamiltonian corresponds to a qutrit . It weakly interacts with a hot () and a cold () bath at inverse temperatures and (as described in Section IV of the main text) to synthesize a bath with negative inverse temperature . The qubit , with Hamiltonian , weakly interacts with a bath with positive inverse temperature . The and interact between them with a time-dependent interaction Hamiltonian given by . After having all these interactions, the overall dynamics of the composite is
| (14) |
for a state , where is the Lindbld super-operator (LOS) representing the dissipative dynamics due to baths and , and is the LOS for the bath with inverse temperature . For this dynamics, the heat flux and power are defined as Alicki (1979); Boukobza and Tannor (2006a)
| (15) | ||||
| (16) |
Note the heat flux and the power may have time dependence.
For time-dependent Hamiltonians, the dynamics generally never leads to a steady state. However, for a periodic time-dependence, as in , there is a rotating frame in which the Hamiltonian can be made time-independent. For that, a counter-rotation is applied on the laboratory frame by with , where . In the rotating frame, an operator in the laboratory frame transforms as . Further, there exists a Hamiltonian for which the interaction Hamiltonian reduces to a time-independent one, given by . Accordingly, the overall Hamiltonian becomes time-independent, and it is . In this rotating frame, the overall dynamics is recast as
| (17) |
for a state , with . Here we denote which is the unitary controbution to the dynamics. This dynamics can lead to a steady state, say . It can be easily checked that the LOSs remain unchanged in this rotating frame. Given that for two arbitrary operators and , we may re-express the heat flux and power as Boukobza and Tannor (2006a)
| (18) |
where , and the power as
| (19) |
where . The heat flux can be divided into two parts. One contribution comes from interaction of with baths and , i.e., and the other due to interaction between with its bath, i.e., .
Now we study the entropy flux through . Note the rate of change in von Neumann entropy is given by
In the rotating frame and at steady-state, it reduces to
The rate of change in entropy vanishes because the state does not change over time. Nevertheless, there still can be a non-vanishing flux of entropy passing through and . Given that unitary dynamics does not contribute to the entropy flux, i.e., , we can calculate the entropy flux through and respectively as
| (20) | |||
| (21) |
where .
Appendix B Power and associated entropy flux in traditional steady-state engines
In traditional engines, unlike heat, work is a form of energy not associated with entropy. To understand that, we may reconsider the heat engine composed of a qutrit and two baths at different temperatures, which is also studied in Boukobza and Tannor (2007). Consider the qutrit system outlined in Section II where the Hamiltonian of the qutrit is , and it is weakly interacting with a hot () and cold () with inverse temperatures and respectively. In addition, an external driving
| (22) |
is introduced. As a result, the reduced dynamics becomes
| (23) |
where , and and are LSOs representing the dissipation due to interactions with hot and cold baths. Again, as discussed in Appendix A, this dynamics leads to a steady-state, say , in a rotating frame. The heat fluxes, entropy fluxes, and power are calculated respectively as
where . Here and represent the heat and entropy fluxes from bath , and is the power. As required by first law, and, at steady-state, . When the device operates as an engine, we have , , and . In this case, heat enters from the hot bath. Part of that heat is converted into work which is stored in the driving field, and the rest is dumped into the cold bath. Further, in this case, and . Clearly, at steady-state, whatever amount of entropy enters the system from the hot bath is released to the cold bath. Thus the work-flux, i.e., power , does not carry any entropy.