Polymer Network Diffusion in Charged Gels
Abstract
The swelling kinetics of charged polymer gels reflect the complex competition among elastic, mixing, and ionic contributions. Here, we used dynamic light scattering to investigate the collective diffusion coefficient of model gels, whose polymer network structure was controlled so that the three contributions were comparable. We demonstrate that the collective diffusion coefficient stems from the sum of elastic, mixing, and ionic contributions, without evident cross-correlations. The significant ionic contribution conforms to the Donnan equilibrium, which explains equilibrium electrical potential gradients in biological systems.
Charged gels, characterized by ionic species immobilized in the polymer network, exhibit enhanced swelling compared to electrically neutral gels because of the excess osmotic pressure caused by the Donnan effect donnan1911theory ; adair1923donnan . Their remarkable ability to swell up to times their dry weight has facilitated their use as superabsorbent polymers for diapers, horticultural water retention agents, and self-healing concrete. This has led to extensive investigation of their swelling behaviors schneider2004discontinuous ; cheng2017preparation ; ricka1984swelling ; jeon1998swelling ; tang2020swelling .
Swelling kinetics are crucial for practical applications of these gels. Tanaka and co-workers tanaka1973spectrum ; tanaka1979kinetics modeled the swelling kinetics of electrically neutral polymer gels, introducing the collective diffusion coefficient of a polymer network as
| (1) |
where is the osmotic bulk modulus, is the shear modulus, and is the friction coefficient (per unit volume) between polymer networks and solvents. However, quantifying based on Eq. (1) remains challenging munch1977inelastic due to difficulties in estimating and . Moreover, is often assumed, leading to tanaka1979kinetics , even though this is an oversimplification.
Equation (1) extends to charged gels by including ionic contribution to from Donnan equilibrium donnan1911theory ; ricka1984swelling ; adair1923donnan . However, validation of this approach is complicated due to the complex competition among polymer-solvent mixing, elastic, and ionic contributions. To date, only a few scaling laws between and the polymer volume fraction have been experimentally examined, including at low salt concentrations skouri1995swelling ; joosten1991dynamic ; raasmark2005fast and at high salt concentrations morozova2017elasticity . The main obstacle in experimental validation arises from the difficulty in controlling polymer networks. Consequently, studies often use natural polymers like xanthan, chitosan, and hyaluronic acid, which lack precise control over network structure, have been typically used, limiting accurate gel dynamics research.
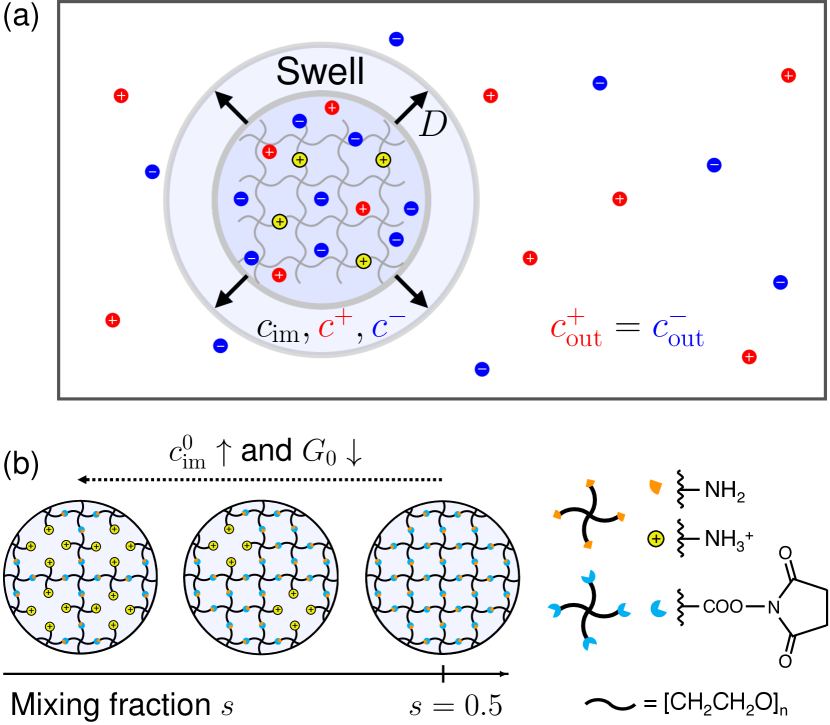
Recently, we have developed a strategy to synthesize tunable model polymer networks sakai2008design and identified the equations governing gel elasticity, including negative energy elasticity yoshikawa2021negative ; sakumichi2021linear , as well as the equation of state of osmotic pressure yasuda2020universal ; sakumichi2022semidilute . We have also quantitatively validated Eq. (1) for neutral gels fujiyabu2018three ; fujiyabu2019shear ; kim2020mixing ; fujiyabu2021temperature , by decomposing into mixing () and elastic () contributions as , where and are expressed by measurable macroscopic properties.
This Letter extends the relationship to charged gels, introducing an ionic contribution :
| (2) |
To validate Eq. (2), we experimentally investigated in charged gels with comparable , , and via dynamic light scattering (DLS). We controlled the molar concentrations of immobilized charge in the gel at the as-prepared state and equilibrated the gels in various outer salt solutions with diverse molar concentrations of mobile ions [Fig. 1(a)]. Our results indicate that the significant contribution of to is explained by the excess osmotic pressure from the Donnan equilibrium donnan1911theory ; adair1923donnan . These findings demonstrate the successful prediction of dynamic property from the static properties of charged gels, suggesting broader applicability of this relationship to charged gels in good solvents.
Theoretical derivation of collective diffusion coefficient in charged gels.—We derive the contribution of to , using a similar procedure as that for and in neutral gels fujiyabu2018three ; fujiyabu2019shear ; kim2020mixing ; fujiyabu2021temperature . The osmotic bulk modulus in Eq. (1) is quantified by total osmotic pressure , where is the polymer mass concentrations. For charged gels, is considered as the sum of polymer-solvent mixing , elastic , and ionic contributions flory1953principles ; flory1943statistical1 ; flory1943statistical2 ; katchalsky1955polyelectrolyte ; katchalsky1951equation ; treloar1973elasticity ; duvsek1993responsive as , where yasuda2020universal ; sakumichi2022semidilute and james1949simple ; horkay2000osmotic . Here, is the excluded volume parameter flory1953principles , and and is the shear modulus and polymer mass concentration, respectively, at the as-prepared state. Further, the friction coefficient per unit volume in Eq. (1) follows fujiyabu2017permeation ; fujiyabu2019shear ; kim2020mixing . Notably, and depend only on at a constant within the same polymer-solvent system, regardless of the polymer network structures yasuda2020universal ; fujiyabu2017permeation ; fujiyabu2019shear ; kim2020mixing . Assuming same dependences of , , and in charged gels, we can express each contribution to using Eq. (1) as
| (3) |
and
| (4) |
The originates from produced by immobilized charge in polymer networks. Notably, Eqs. (3) have been validated for neutral gels at as-prepared states fujiyabu2018three ; fujiyabu2019shear ; fujiyabu2021temperature and swollen states kim2020mixing .
We quantified using the Donnan equilibrium donnan1911theory ; ricka1984swelling ; adair1923donnan . For a polymer network with immobilized charge molar concentration in a solution containing monovalent ions, the molar concentrations of mobile cations and anions inside the gel ( and ) and in the outer salt solution ( and ) satisfy the following electric neutrality conditions: (i) for the inner gel, (ii) for the outer salt solution, and (iii) between inner gel and outer salt solution. Combining the conditions (i) and (iii) yields , where () is the molar concentration ratio between the mobile ion in the outer salt solution and the immobilized charge in the polymer network. Hence, the osmotic pressure due to the immobilized charge is
| (5) |
Substituting Eq. (5) into Eq. (4) gives
| (6) |
According to Eq. (6), regulates the efficiency of , with in the charged gel limit () and in the neutral gel limit ().
Materials and methods.—As a model system to investigate charged gels, we used a tetra-arm poly(ethylene glycol) (PEG) hydrogel, synthesized via the AB-type cross-end coupling of two prepolymers (tetra-arm PEG) of equal size. Each end of the tetra-arm PEG was modified with a mutually reactive amine (tetra-PEG-NH2) and succinimidyl ester (tetra-PEG-OSu) (NOF Co., Tokyo, Japan). All other reagents were purchased from WAKO Pure Chemicals (Osaka, Japan). All materials were used without further purification. We dissolved tetra-PEG-NH2 and tetra-PEG-OSu (molar mass kgmol) in phosphate buffer (, mM) to achieve a concentration of gL.
To fabricate charged gels, we mixed two solutions at stoichiometrically imbalanced ratios of , , , and (Table 1), where is the molar fraction of minor tetra-PEG polymers ([tetra-PEG-OSu]) to total tetra-PEG polymers ([tetra-PEG-NH2] [tetra-PEG-OSu]). An excess tetra-PEG-NH2 () allowed some amine groups to remain partly unreacted yoshikawa2019connectivity , serving as the immobilized cations in the polymer networks [see Fig. 1(b)]. We kept each sample in an enclosed space to maintain humid conditions at room temperature () for reaction completion.
We immersed each charged gel into the outer salt (HCl NaCl) solutions of and – mM (Table 1) to completely protonate immobilized cations and to tune the parameter in Eq. (6). We replaced the outer salt solution twice every hours to achieve equilibrium swollen state. The Henderson-Hasselbalch equation henderson1908concerning ; hasselbalch1917calculation determined the dissociation constant of immobilized residues as M (see Supplemental Material, Sec. S1 supplement ), confirming complete protonation of amines in all experimental conditions because was smaller than [H+] M.
We measured the diameter of each gel at equilibrium in the outer salt solutions, using an optical microscope (M165 C; Leica, Wetzlar, Germany) to calculate the volume swelling ratio (initial diameter mm), determining and . To ensure accurate and values at each , we assessed the sol fraction eluted out from the polymer network during the swelling process using the Bethe approximation supplement ; macosko1976new ; miller1976new (Further details are described in Supplemental Material, Sec. S2 supplement ).
| (gL) | (mM) | (mM) | (kPa) | |||
|---|---|---|---|---|---|---|
We measured of charged gels via DLS (ALV/CGS-3 compact goniometer, Langen, Germany) in the same way as Refs. fujiyabu2018three ; fujiyabu2019shear ; kim2020mixing ; fujiyabu2021temperature . Each gel sample was fabricated in a scattering glass tube (disposable culture tube 9830-1007 with an inner diameter of mm; IWAKI, Japan) and was swollen in the outer salt solution. We carefully selected of each gel to prevent contact between a swollen gel and an inner wall of the glass tube. Once equilibrium was reached, we measured the scattering light intensity at time over s at a scattering wavelength nm and a scattering angle , to evaluate the autocorrelation function johnson1994laser ; berne2000dynamic for delay time – ms, corresponding to the thermal fluctuation of a polymer network li2017probe ; fujiyabu2018three ; fujiyabu2019shear ; kim2020mixing ; fujiyabu2021temperature ; ohira2018dynamics (results are provided in Supplemental Material, Sec. S3 supplement ). Here, denotes the time-average. We fitted the autocorrelation functions using a stretched exponential function , where is the initial amplitude, is the stretched exponent, and is the time-independent background. Using the partial heterodyne model joosten1991dynamic ; shibayama2002gel , we evaluate , where nm-1 is the scattering vector with the refractive index of the aqueous solvents.
We measured the (equilibrium) shear modulus of gels at the equilibrium swollen state for each using a rheometer (MCR302; Anton Paar, Graz, Austria) with a -mm parallel plate geometry. The gels, shaped into discs with a height mm and a diameter mm, were equilibrated in outer salt solutions with varying (Table 1). We measured the storage modulus and loss modulus at K with angular frequency – rads and shear strain %, as well as rads and % –%. For all samples, was independent of and , and was much larger than (Supplemental Material, Sec. S4 supplement ). Hence, we selected measured at rads and % as the (equilibrium) shear modulus . Using for swollen gels james1949simple ; horkay2000osmotic (see Supplemental Material, Sec. S5 supplement ), we evaluated at the as-prepared state as for each (Table 1).
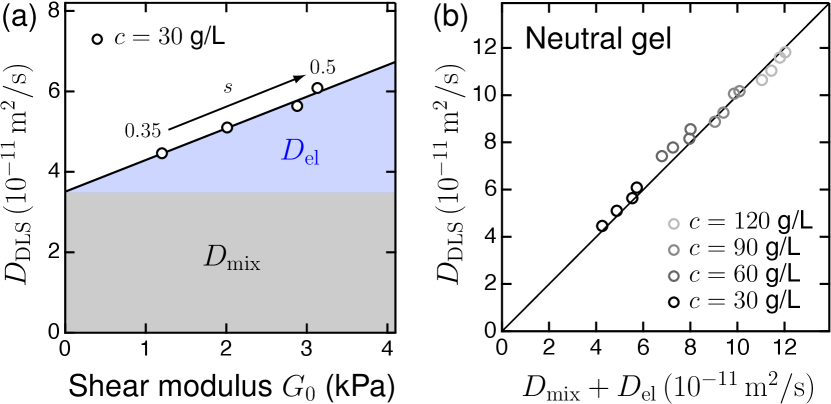
Estimating and in neutral gels.—Before considering charged gels, we briefly revisit the decomposition of mixing () and elastic () contributions to in a model neutral gels, based on our previous studies fujiyabu2018three ; fujiyabu2019shear ; kim2020mixing ; fujiyabu2021temperature . Figure 2(a) shows the collective diffusion coefficient measured via DLS in neutral gels at the as-prepared state, plotted against for various . The observed linear relationship between and is consistent with Eqs. (3). Notably, is independent of , because and is independent of yasuda2020universal ; fujiyabu2017permeation ; fujiyabu2019shear ; kim2020mixing . Thus, we can decompose into the mixing contribution and the elastic contribution [see Fig. 2(a)].
Through the decomposition of and , we obtain the empirical formulas and for neutral gels (the detailed results are provided in Supplemental Material, Sec. S7), corroborating the scaling predictions tokita1991friction and des1975lagrangian ; de1979scaling (for gels in a good solvent). These formulas are applicable to neutral gels at the as-prepared and swollen states, as confirmed in our previous study kim2020mixing . Using values of and , we can accurately calculate for a neutral gel. Figure 2(b) demonstrates that the collective diffusion coefficient of neutral gels measured via DLS () shows excellent agreement with Eqs. (3), using empirical formulas of and .
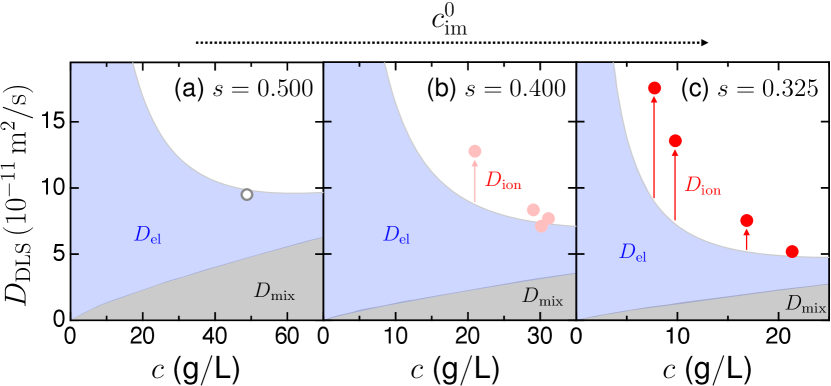
Estimating in charged gels.— We expand our analysis to electrically charged gels and examine Eq. (2), by hypothesizing that the formulas of and , as established for neutral gels, remain applicable. Figure 3 shows for swollen neutral and charged gels (equilibrated in outer salt solutions with varying ), as measured via DLS. For varying samples, a decrease in corresponded with an increase in and a decrease in . This resulted in an increase in to be a decrease in () in outer salt solutions (Table 1). The decrease in is attributed to a decrease in elastically effective subchains in polymer networks sakai2008design . Moreover, for a fixed , a decrease in in outer salt solutions led to a lower (), resulting in increased to be decreased () (Table 1).
For neutral gels [Fig. 3(a)], agrees with , which is independent of (see Supplemental Material, Sec. S8 supplement for further details). For charged gels with each [Fig. 3(b) and (c)], we calculate (gray region) and (blue region), using the same empirical formulas for neutral gels with all measurable and . Here, we experimentally confirmed that holds for charged gels (see Supplemental Material, Sec. S5 supplement ), indicating that the immobilized charge does not significantly affect the elastic modulus. At high (), approaches the neutral gel limit (), because in Eq. (6). In contrast, at low (), deviates from the neutral gel limit (), indicating a significant to (indicated by red arrows), which contributed up to approximately half of at its maximum.
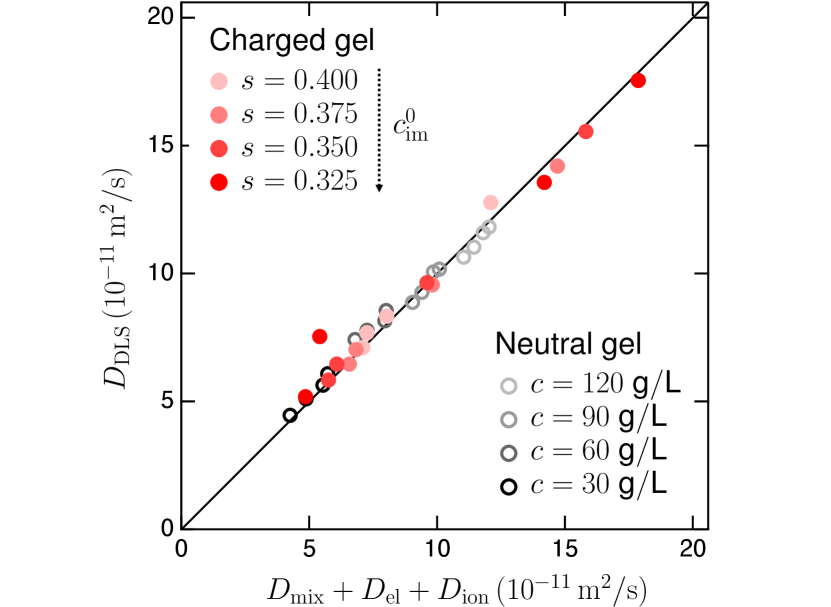
We show that of charged gels can be accurately described by simply adding in Eq. (6). Figure 4 demonstrates that the collective diffusion coefficient of charged gels measured via DLS () shows excellent agreement with the predictions of Eqs. (3) and (4), using the same empirical formulas of and as in neutral, and applying Eq. (6) with each and . These findings suggest that Eq. (1) can be extended to include charged gels by simply adding without evident cross-correlations. Notably, Eq. (6) is applicable in weakly charged gels and is less effective in polyelectrolyte gels with high , due to counterion condensation tang2020swelling ; manning1969limiting .
Concluding remarks.— We developed model charged gels [Fig. 1 and Table 1] with comparable and and measured their collective diffusion coefficient via DLS. Based on the same decomposition of and to in neutral gels [Figs. 2 and 3], our results indicate that in the charged gel is governed by Eq. (1), where emerges as an additive component [Fig. 4]. The significant ionic contribution to is quantitatively described through the ionic osmotic pressure [Eqs. (5) and (6)], which originates from the Donnan equilibrium.
Our findings demonstrate the successful prediction of the dynamic behaviors from the static properties of charged gels equilibrated in a good solvent. This aspect is particularly relevant considering the dynamics in gel-like biological systems such as extracellular matrices, which are predominantly negatively charged theocharis2016extracellular and influenced by the Donnan effect. Therefore, an in-depth understanding of the dynamics in charged gels will enhance our comprehension of the behaviors of biological systems.
Acknowledgements.
This work was supported by the Japan Society for the Promotion of Science (JSPS) through the Grants-in-Aid for JSPS Research Fellows Grant No. 19J22561 to T.F. and No. 202214177 to T.Y., Scientific Research (B) Grant No. 22H01187 to N.S., Scientific Research (A) Grant No. 21H04688 to T.S., Transformative Research Area Grant No. 20H05733 to T.S., and MEXT Program Grant No. JPMXP1122714694 to T.S. This work was also supported by JST through CREST Grant No. JPMJCR1992 and Moon-shot R&D Grant No. 1125941 to T.S.References
- (1) F. G. Donnan, Theory of the balances of membranes and potential of membranes at the existence of non dialysing electrolytes-A contribution to physical chemical physiology, Z. Elektrochem. Angew. Phys. Chem. 17, 572 (1911).
- (2) G. S. Adair, On the Donnan equilibrium and the equation of Gibbs, Science 58, 13 (1923).
- (3) J. Ricka and T. Tanaka, Swelling of ionic gels: quantitative performance of the Donnan theory, Macromolecules 17, 2916 (1984).
- (4) C. H. Jeon, E. E. Makhaeva, and A. R. Khokhlov, Swelling behavior of polyelectrolyte gels in the presence of salts, Macromol. Chem. Phys. 199, 2665 (1998).
- (5) S. Schneider and P. Linse, Discontinuous volume transitions in cross-linked polyelectrolyte gels induced by short-range attractions and strong electrostatic coupling, Macromolecules 37, 3850 (2004).
- (6) W. M. Cheng, X. M. Hu, Y. Y. Zhao, M. Y. Wu, Z. X. Hu, and X. T. Yu, Preparation and swelling properties of poly (acrylic acid-co-acrylamide) composite hydrogels, e-Polym. 17, 95 (2017).
- (7) J. Tang, T. Katashima, X. Li, Y. Mitsukami, Y. Yokoyama, N. Sakumichi, U. Chung, M. Shibayama, and T. Sakai, Swelling behaviors of hydrogels with alternating neutral/highly charged sequences, Macromolecules 53, 8244 (2020).
- (8) T. Tanaka, L. O. Hocker, and G. B. Benedek, Spectrum of light scattered from a viscoelastic gel, J. Chem. Phys. 59, 5151 (1973).
- (9) T. Tanaka and D. J. Fillmore, Kinetics of swelling of gels, J. Chem. Phys. 70, 1214 (1979).
- (10) J. P. Munch, S. Candau, J. Herz, and G. Hild, Inelastic light scattering by gel modes in semi-dilute polymer solutions and permanent networks at equilibrium swollen state, J. Phys. 38, 971 (1977).
- (11) R. Skouri, F. Schosseler, J. P. Munch, and S. J. Candau, Swelling and elastic properties of polyelectrolyte gels, Macromolecules 28, 197 (1995).
- (12) J. G. H. Joosten, J. L. McCarthy, and P. N. Pusey, Dynamic and static light scattering by aqueous polyacrylamide gels, Macromolecules 24, 6690 (1991).
- (13) P. J. Råsmark, Č. Koňák, and P. Štěpánek, and C. Elvingson, Fast internal dynamics in polyelectrolyte gels measured by dynamic light scattering, Polym. Bull. 54, 335 (2005).
- (14) S. Morozova and M. Muthukumar, Elasticity at swelling equilibrium of ultrasoft polyelectrolyte gels: Comparisons of theory and experiments, Macromolecules 50, 2456 (2017).
- (15) T. Sakai, T. Matsunaga, Y. Yamamoto, C. Ito, R. Yoshida, S. Suzuki, N. Sasaki, M. Shibayama, and U. Chung, Design and fabrication of a high-strength hydrogel with ideally homogeneous network structure from tetrahedron-like macromonomers, Macromolecules 41, 5379 (2008).
- (16) Y. Yoshikawa, N. Sakumichi, U. I. Chung, and T. Sakai, Negative Energy Elasticity in a Rubberlike Gel, Phys. Rev. X 11, 011045 (2021).
- (17) N. Sakumichi, Y. Yoshikawa, and T. Sakai, Linear elasticity of polymer gels in terms of negative energy elasticity, Polym. J. 53, 1293 (2021).
- (18) T. Yasuda, N. Sakumichi, U. Chung, and T. Sakai, Universal equation of state describes osmotic pressure throughout gelation process, Phys. Rev. Lett. 125, 267801 (2020).
- (19) N. Sakumichi, T. Yasuda, and T. Sakai, Semidilute Principle for Gels, arXiv:2210.15275.
- (20) T. Fujiyabu, F. Toni, X. Li, U. Chung, and T. Sakai, Three cooperative diffusion coefficients describing dynamics of polymer gels, Chem. Commun. 54, 6784 (2018).
- (21) T. Fujiyabu, Y. Yoshikawa, J. Kim, N. Sakumichi, U. Chung, and T. Sakai, Shear modulus dependence of the diffusion coefficient of a polymer network, Macromolecules 52, 9613 (2019).
- (22) J. Kim, T. Fujiyabu, N. Sakumichi, T. Katashima, Y. Yoshikawa, U. Chung, and T. Sakai, Mixing and elastic contributions to the diffusion coefficient of polymer networks, Macromolecules 53, 7717 (2020).
- (23) T. Fujiyabu, T. Sakai, R. Kudo, Y. Yoshikawa, T. Katashima, U. Chung, and N. Sakumichi, Temperature dependence of polymer network diffusion, Phys. Rev. Lett. 127, 237801 (2021).
- (24) P. J. Flory, Principles of polymer chemistry (Cornell University Press, Ithaca, 1953).
- (25) P. J. Flory and J. Rehner, Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity., J. Chem. Phys. 11, 512 (1943).
- (26) P. J. Flory and J. Rehner, Statistical mechanics of cross-linked polymer networks II. Swelling. J. Chem. Phys., J. Chem. Phys. 11, 521 (1943).
- (27) A. Katchalsky, S. Lifson, and H. Exsenberg, Equation of swelling for polyelectrolyte gels, J. Polym. Sci. 7, 571 (1951).
- (28) A. Katchalsky and I. Michaeli, Polyelectrolyte gels in salt solutions, J. Polym. Sci. 15, 69 (1955).
- (29) L. R. G. Treloar, The elasticity and related properties of rubbers, Reports Prog. Phys. 36, 755 (1973).
- (30) K. Dušek and M. Ilavskỳ, Responsive gels: volume transitions I (Springer, Berlin, 1993).
- (31) H. M. James and E. Guth, Simple Presentation of Network Theory of Rubber, with a Discussion of Other Theories. J. Polym. Sci. 4, 153 (1949).
- (32) F. Horkay, I. Tasaki, and P. J. Basser, Osmotic Swelling of Polyacrylate Hydrogels in Physiological Salt Solutions. Biomacromolecules 1, 84 (2000).
- (33) T. Fujiyabu, X. Li, M. Shibayama, U. Chung, and T. Sakai, Permeation of water through hydrogels with controlled network structure. Macromolecules 50, 9411 (2017).
- (34) Y. Yoshikawa, N. Sakumichi, U. Chung, and T. Sakai, Connectivity dependence of gelation and elasticity in AB-type polymerization: an experimental comparison of the dynamic process and stoichiometrically imbalanced mixing, Soft Matter 15, 5017 (2019).
- (35) L. J. Henderson, Concerning the relationship between the strength of acids and their capacity to preserve neutrality, Am. J. Physiol. Content 21, 173 (1908).
- (36) K. A. Hasselbalch, The calculation of the hydrogen content in blood from free and combined carbonic acid, and the oxygen compound of the blood as the function of the hydrogen content, Biochem. Z. 78, 112 (1917).
- (37) See Supplemental Material at [URL] for details of experiments and analyses, including calculations.
- (38) C. W. Macosko and D. R. Miller, A new derivation of average molecular weights of nonlinear polymers, Macromolecules 9, 199 (1976).
- (39) D. R. Miller and C. W. Macosko, A new derivation of post gel properties of network polymers, Macromolecules 9, 206 (1976).
- (40) C. S. Johnson and D. A. Gabriel, Laser light scattering (Dover Publications, Mineola, 1994).
- (41) B. J. Berne and R. Pecora, Dynamic light scattering: with applications to chemistry, biology, and physics (Dover Publications, Mineola, 2000).
- (42) M. Ohira, X. Li, C. I. Gupit, H. Kamata, T. Sakai, and M. Shibayama, Dynamics of thermoresponsive conetwork gels composed of poly(ethylene glycol) and poly(ethyl glycidyl ether-co-methyl glycidyl ether), Polymer 155, 75 (2018).
- (43) X. Li, N. Watanabe, T. Sakai, and M. Shibayama, Probe diffusion of sol-gel transition in an isorefractive polymer solution, Macromolecules 50, 2916 (2017).
- (44) M. Shibayama and T. Norisuye, Gel formation analyses by dynamic light scattering, Bull. Chem. Soc. Jpn. 75, 641 (2002).
- (45) M. Tokita and T. Tanaka, Friction coefficient of polymer networks of gels, J. Chem. Phys. 95, 4613 (1991).
- (46) J. des Cloizeaux, The Lagrangian theory of polymer solu- tions at intermediate concentrations, J. Phys. (Paris) 36, 281 (1975).
- (47) P. G. de Gennes, Scaling Concepts in Polymer Physics (Cornell University Press, Ithaca, 1979).
- (48) G. S. Manning, Limiting laws and counterion condensation in polyelectrolyte solutions I. Colligative properties, J. Chem. Phys. 51, 924 (1969).
- (49) A. D. Theocharis, S. S. Skandalis, C. Gialeli, N. K. Karamomos, Extracellular matrix structure, Adv. Drug Delivery Rev. 97, 4 (2016).