Hydraulic and electric control of cell spheroids
Abstract
We use a theoretical approach to examine the effect of a radial fluid flow or electric current on the growth and homeostasis of a cell spheroid. Such conditions may be generated by a drain of micrometric diameter. To perform this analysis, we describe the tissue as a continuum. We include active mechanical, electric, and hydraulic components in the tissue material properties. We consider a spherical geometry and study the effect of the drain on the dynamics of the cell aggregate. We show that a steady fluid flow or electric current imposed by the drain could be able to significantly change the spheroid long-time state. In particular, our work suggests that a growing spheroid can systematically be driven to a shrinking state if an appropriate external field is applied. Order-of-magnitude estimates suggest that such fields are of the order of the indigenous ones. Similarities and differences with the case of tumors and embryo development are briefly discussed.
Introduction
Understanding how cells collectively organize to form complex structures and organs is the fundamental question raised in morphogenesis. This self-organization stems from the interplay of biochemical Green and Sharpe (2015); Miller and Davidson (2013), mechanical Miller and Davidson (2013); Mammoto et al. (2013); Ladoux and Mège (2017), but also hydraulic Ruiz-Herrero et al. (2017); Dumortier et al. (2019) and electrical processes Levin and Martyniuk (2018); Silver and Nelson (2018). A long-standing paradigm in developmental biology is that cell chemical signals, in the form of morphogens, control cell growth and differentiation leading to tissue patterning Green and Sharpe (2015). Although this biochemical signaling is of paramount importance in developmental control, it is now well-established that mechanical forces between cells or mediated by the extra-cellular matrix can also provide regulatory cues that are equally important Mammoto et al. (2013).
The crucial importance of hydraulics in morphogenesis, which should not come as a surprise given the large water content in tissues, has also been highlighted in multiple experiments. Hydraulic oscillations have for instance been shown to provide a robust mechanism for size control of the mouse embryo Chan et al. (2019) and during the Hydra regeneration Fütterer et al. (2003). The role of electrical signals in tissue patterning, although already studied by Roux Roux (1892) at the end of the 19th century, has gained a new interest only recently McCaig et al. (2005); Levin and Martyniuk (2018); McLaughlin and Levin (2018). In addition to its key function in receiving and relaying sensory information in the nervous system Watanabe et al. (2009), bioelectricity has been shown to have a dramatic importance in large-scale patterning: an alteration of the electrical signaling in Planaria regeneration causes for instance the emergence of animals with multiple heads Levin and Martyniuk (2018). Similarly, it has recently been observed that an external electric field can be used to reverse the morphogenetic fate of Hydra Braun and Ori (2019).
In the quest of understanding how these different mechanisms come together to shape tissues and organs, simple cell aggregates such as spheroids have offered an appealing territory to observe tissue development and to formulate hypotheses on the underlying mechanisms. Remarkably, a rich behavior is observed even in single-cell type spheroids, as for instance their ability to pump fluid and form liquid-filled lumens Martín-Belmonte et al. (2008); Debnath et al. (2002). More recently, organoids have focused an increasing attention as they can recapitulate complex morphogenetic processes in a relatively simple and controllable environment Huch and Koo (2015); Dahl-Jensen and Grapin-Botton (2017).
To unravel the connections between biochemical signals and tissue mechanics, mechanical perturbations of organoids and cell spheroids can be performed. For instance, atomic force microscopy has been used to probe the mechanical properties of mammary organoids Alcaraz et al. (2008) and revealed the importance of both extracellular matrix stiffness and laminin signaling to maintain tissue integrity. Perturbation of the osmotic pressure around cell spheroids using large molecules of Dextran has also highlighted the importance of isotropic stress in tissue growth Montel et al. (2011); Delarue et al. (2013).
A broad understanding of tissue mechanics therefore requires to consider tissue electrohydraulic properties and to be able to perturb tissues by electric or hydraulic means. In this theoretical work, we study the response of a cell spheroid to electric and hydraulic perturbations. We propose an experimental setup where a pipette or drain is used to impose a fluid flow or an external electric current through a micrometric drain. Our work shows that one could control the size of a cellular assembly using such a setup.
Our theoretical approach relies on generic features of the physics of the electro-hydrodynamic phenomena at play within tissues. We adopt a coarse-grained approach of tissues, which describes many cells and their micro-environments as a continuum with active material properties Kruse et al. (2005); Marchetti et al. (2013); Ranft et al. (2010). Cell mechanical characteristics, fluid pumping, ion transport and electrical properties are thus considered in a unified framework Sarkar et al. (2019); Duclut et al. (2019) that we use to analyze spheroid response to an external perturbation.
In particular, we highlight in the following that steady external flow or electric current imposed by a drain can systematically drive a proliferating spheroid to degeneracy. Such technique could be relevant in a medical context where it could be used to suppress cancerous tumors.
Methods for suppressing malignant tumors are numerous: a first path to control tumor size is to use drugs to disturb the chemical regulation of cancerous cells to prevent them from proliferating (chemotherapy). Immunotherapy has offered a strong alternative in mitigating cancer by stimulating the immune system to suppress tumors Scott et al. (2012). In addition, radiotherapy – which allows the suppression of the tumor by damaging the genetic material of cancerous cells through radiations – has also proved effective for tumor removal Baskar et al. (2012).
Physics-based methods can also be used to provide new techniques for directly suppressing the cancerous tissues. High-intensity focused ultrasounds can for instance be used to locally overheat the cancerous cells Al-Bataineh et al. (2012). Recent experiments also suggest that lower intensity ultrasound waves could be used to strain and suppress mechanically cancerous tissues Mittelstein et al. (2020); Tijore et al. (2020), and low-frequency ultrasounds could be used to increase selectivity Tijore et al. (2020). Electrical perturbations have also been used: cancerous tissues can for instance be successfully suppressed using irreversible electroporation Miller et al. (2005) by applying large voltage pulses in the tissue. More recently, micro-electrodes have been used in electrolytic ablation methods to locally change pH and kill a cancerous mass Perkons et al. (2018).
Compared to other treatments and ablation techniques Knavel and Brace (2013), direct electrohydraulic perturbations of cancerous tissues have however remained largely unexplored. With the theoretical study that we present here, we aim at highlighting potential novel methods for controlling size of cell spheroids, that could also be used to suppress cancerous tumors.
I Continuum model of a spheroid with a drain


Following Refs. Sarkar et al. (2019); Duclut et al. (2019), we consider the tissue at a coarse-grained level, such that individual cells are not described but the tissue as a whole is studied as a continuum material with active electrical, hydraulic and mechanical properties. To capture the hydraulic properties of the tissue, we also adopt a two-fluid description, where the cells, which form the first fluid, are permeated by the interstitial fluid Ranft et al. (2012). In the long-time limit (several days or weeks) that we consider here, cells are able to reorganize and to relax the internal stresses within the tissue, such that an effective description of the tissue as a viscous active fluid at long times is used Ranft et al. (2010).
To analyze specifically the effect of a drain on a cell spheroid, we consider a spherical tissue of radius enclosing a spherical lumen of radius (see Fig. 1). A drain of inner radius is inserted inside the spheroid and can be used to impose an external flow or an external current. We consider, despite the presence of the drain, a system with spherical symmetry. The description we propose in the following is therefore effectively one dimensional and depends only on the distance to the spheroid center.
Directional ion pumping through the spheroid is achieved if cells have a polarity. We thus define a cell polarity field with unit norm that we assume, for simplicity, to be oriented along the radial direction: . Cells also display a nematic ordering – due for instance to an anisotropy in their shape – that we describe with the nematic tensor (Greek indices indicate Cartesian coordinates). We assume in the following that the nematic ordering is defined by the same preferred axis as the polarity of the cells: . This situation is for instance observed in colon carcinoma cell spheroids Dolega et al. (2017). Finally, the consideration of ion pumping within the tissue requires the introduction of an electric field and an electric current density , which obey a generalized Ohm law as we discuss in the following.
I.1 Tissue mechanical, hydraulic and electrical properties
The total tissue stress is decomposed as where here and in the following the superscripts stand for the cells and interstitial fluid contributions, respectively. Neglecting inertia and in the absence of external bulk forces, force balance within the tissue reads:
| (1a) | ||||
| (1b) | ||||
where represents internal forces between the cells and the interstitial fluid, and where summation over repeated indices is implied.
To obtain the dynamics of the spheroid, that is, the time evolution of the inner and outer radii and , we now need to specify the material properties (or constitutive equations) of the tissue. In our coarse-grained description, the interstitial fluid is driven by pressure gradients, while fluid viscosity contributes to the internal forces between fluid and cells. We therefore write the fluid stress as . For the cell stress, the description of the material properties is made easier by decomposing the stress into a traceless symmetric part and an isotropic part . The constitutive equation for the isotropic part then reads Sarkar et al. (2019); Duclut et al. (2019):
| (2a) | |||
| where is the local pressure and we have introduced the cell strain-rate tensor and where . The bulk viscosity is a familiar term that is also present for a passive fluid, except that its microscopic origin is different for a tissue. The homeostatic pressure is a property specific for tissues that results from a balance of cell growth and cell death Basan et al. (2009). In addition to these terms, we have included additional terms in the isotropic stress: cell anisotropies can couple to the anisotropic stress, resulting in the term proportional to . The terms proportional to and represent bioelectric and biohydraulic stresses induced by a coupling to the electric field or by a (relative) fluid flow, respectively. A similar expansion for the traceless anisotropic part of the stress tensor reads: | |||
| (2b) | |||
| where is the traceless part of the cell strain-rate tensor, and we have moreover defined the symmetric traceless part of a dyadic product of vectors: . The coefficient is the usual shear viscosity while the other terms represent active couplings, and the terms proportional to and are the anisotropic counterpart of the terms proportional to and in Eq. (2a). The active stress is a hallmark of active systems and shows the ability of cells to generate anisotropic stresses due to cell division or contraction of their cytoskeleton Simha and Ramaswamy (2002); Bittig et al. (2008). This active stress can be regulated by the cells and we therefore consider that it depends on the local pressure at linear order as: | |||
| (2c) | |||
We emphasize that the cell stress constitutive equations (Eqs. (2a) to (2c)) reflect the effective viscous properties of tissues at long time as a consequence of cellular growth and death. We review the derivation of these constitutive equations in App. A. A key feature of our work is that flows and electric fields do influence cell division and death, and therefore growth and shrinkage of the spheroid Blackiston et al. (2009); Sarkar et al. (2019); Duclut et al. (2019).
The constitutive equation for the internal force density between cells and interstitial fluid including all the linear terms allowed by symmetry can be written as111Note that additional terms must be added to Eqs. (3) and (4) for a system lacking spherical symmetry or for a system where the polarity is not purely radial with unit norm, see App. A.3 for details.
| (3) |
The first term accounts for the friction between the interstitial fluid and the cells and leads to Darcy’s law Darcy (1856) in the description of porous materials. The permeation coefficient can be estimated as , where is the interstitial fluid viscosity and a typical interstitial distance. The term accounts for active fluid pumping by the cells, while the terms proportional to correspond to the electroosmotic contribution. The last term, proportional to , characterizes a differential pumping term due to the bending of the cells Ramaswamy et al. (2000).
To complete the tissue properties description, one finally needs to specify the constitutive equation for the electric current density222Note that additional terms must be added to Eqs. (3) and (4) for a system lacking spherical symmetry or for a system where the polarity is not purely radial with unit norm, see App. A.3 for details.
| (4) |
where is the electric conductivity of the tissue. The term proportional to characterizes the current due to the (relative) flow of ions between cells as a consequence of a reverse electroosmotic effect Kirby (2013). The coefficient characterizes the contribution of ion pumping to the electric current, while the coefficient is an active flexoelectric coefficient. It indicates that a spatially nonuniform cell polarity orientation is obtained in response to an electric field, and has been shown to play a crucial role in the nucleation of a lumen in spherical cell aggregates Duclut et al. (2019).
I.2 Drain description
The drain can be used to impose an external fluid flow or an external electric current in two equivalent ways: either by imposing directly a volumetric flow rate and electric current through the drain, or, alternatively, a pressure difference (with the pressure at the outer spheroid boundary and the pressure at the outer end of the drain, see Fig. 1) and an electric potential difference (with the electric potential at outer spheroid boundary and the electric potential at the outer end of the drain) can be applied.
In both cases, the imposed external fluid flow and external electric current density at the lumen boundary read:
| (5) |
where and are the fluid velocity and electric current density at the boundary between the spheroid and the lumen.
In the following, we focus on the case where and are imposed externally. The case of a pressure difference or electric potential difference imposed by the drain are discussed in App. C.
I.3 Continuity equations and boundary conditions
If cell density and interstitial fluid density are equal and constant, which we assume in the following, then the total volume flux is divergence-free: (see App. A for details). In the presence of the drain, which imposes a nonvanishing fluid velocity at the inner boundary of the spheroid, the integration of the total flow incompressibility in spherical coordinates yields a relation between the fluid velocity and cell velocity in the tissue, which reads:
| (6) |
Similarly, charge conservation in the quasistatic limit can be integrated in the case where an external current density is imposed on the inner boundary, yielding the current density throughout the tissue.
The spheroid is surrounded by an external fluid both inside (in the lumen), and outside. This fluid exerts a hydrostatic pressure on the tissue that is balanced by the tissue surface tension and by the total normal stress at the boundaries:
| (7a) | ||||
| (7b) | ||||
where we have introduced the inner and outer tissue surface tensions and . Fluid exchange between the spheroid and the outside is driven by osmotic conditions:
| (8a) | ||||
| (8b) | ||||
Here, are the permeability of the interfaces to water flow. The fluxes and can be nonzero as a result of active pumps and transporters that maintain an osmotic pressure difference, and act effectively as water pumps. We have also introduced , the external flow imposed at outer spheroid boundary. Conservation of the volumetric flow directly yields .
The normal velocity of the cells at the boundaries has to match the growth of the spheroid radii. An increased cell proliferation in a thin surface layer has been observed in growing spheroids Delarue et al. (2014); Montel et al. (2011); Delarue et al. (2013). We thus allow for a thin surface layer of cells, both facing outside and to the lumen, to have a growth rate that differs from the bulk. The cell velocity boundary conditions then read:
| (9a) | |||
| (9b) | |||
where with the thickness of the boundary layers, and the cell number density and the cell growth rate in the surface layers, respectively.
II Dynamics of the spheroid growth and orders of magnitude
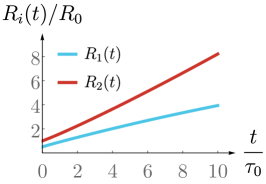



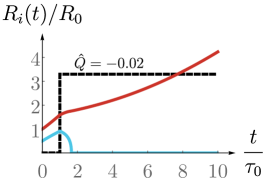

We have introduced in the previous section a model for a spherical spheroid with a drain. Solving force balance (1) together with the boundary conditions (7)-(9) then allow us to obtain the dynamics of the inner and outer radii of the spheroid in the quasistatic limit. We obtain two coupled nonlinear differential equations for the spheroid radii, Eqs. (38a) and (38b) (see App. B), which have been studied in Ref. Duclut et al. (2019) in the absence of a drain. As we will see in the following, imposing an external fluid flow or electric current have dramatic consequences for the spheroid growth and can be used to control its size.
Before discussing how the presence of external fields modify the dynamics of a spheroid and which protocols can be used to control its growth, we first use our model to discuss the orders of magnitude of the external flux and electric current for which we expect a significant change in the spheroid dynamics. To obtain these estimates, we assume that the lumen size is small and use in Eq. (38b) that describes the dynamics of the spheroid. We then compare in this equation the stresses generated by imposed flows or electric currents to the stresses stemming from internal activity in the absence of flows and currents. We find that the external volumetric flow required to significantly perturb the spheroid is of the order:
| (10) |
where is an effective permeation coefficient and is a typical scale of tissue stress in the absence of flows and currents. Note that the appearance of in Eq. (10) indicates that the finite bulk permeability of the spheroid governs the effects of the external flow imposed via the drain. Note however that for smaller spheroids of size m, the flow-induced effects are not dominated by bulk permeation but rather by surface permeation and we have: . We can estimate the external electric current required to perturb the spheroid:
| (11) |
One can use experimental values and order-of-magnitude estimates of the parameters that appear in Eqs. (10) and (11) (see Table 1). We estimate that a volumetric flow m3/s is sufficient to observe a significant change in the dynamics of a millimetre-sized spheroid, and this volumetric flow scales linearly with the size of the spheroid. Similarly, electric current of the order nA can perturb the dynamics of a millimetre-sized spheroid (see also Fig. 6 in App. E).
Externally imposed flows and electric currents could therefore be used to induce a change in the spheroid behavior. In the following sections, we focus in more details on how these external perturbations can be used to control the size of the spheroid.
| Parameters | Exp. values | Parameters | Estimations Sarkar et al. (2019); Duclut et al. (2019) |
|---|---|---|---|
| Forgacs et al. (1998) | Pas | Asm3 | |
| Montel et al. (2011) | Pas | Nm3 | |
| Forgacs et al. (1998) | N/m | NmV | |
| Netti et al. (2000) | m2/Pa/s | Nm2 | |
| Brace and Guyton (1977) | Pa | Am2 | |
| Delarue et al. (2014) | m/s | AV/m | |
| Montel et al. (2011) | Pa | Am | |
| Delarue et al. (2014) | Pa | N/m/V | |
| Delarue et al. (2014) | Pas/m | ||
| m | N/m/V | ||
| Pas/m | |||
| m/Pa/s | |||
| m/s | |||
III Hydraulic and electric control of the size of a spheroid
III.1 Examples of protocols for spheroid suppression
Using our model and solving numerically Eqs. (38a) and (38b) (see App. D), we can analyse various protocols for the suppression of a spheroid. The dynamics of the spheroid and its lumen, and , is therefore studied for different external flow protocols and electric current protocols . To keep the discussion as general as possible, we introduce dimensionless quantities: a dimensionless radius , time , external volumetric flow and electric current , where we have defined:
| (12) |
Note that the effective pressure introduced above is a modification of the homeostatic pressure by the external osmotic pressure and by electric and active contributions. Its expression can be found in Table 2 and in App. B.
We display in Fig. 2 different protocols that can be applied to a growing spheroid in order to suppress it. If the external flow magnitude is sufficiently large and if it is applied long enough, the interventions are successful and lead to the spheroid suppression. Figure 2(b) shows an example of a successful suppression of the spheroid as a sufficiently strong flow has been applied until the spheroid is suppressed. A larger flow magnitude leads to a faster spheroid suppression (Fig. 2(c)). We also show in Fig. 2(d) an example of successful suppression protocol for which the magnitude of the flow is lowered as the spheroid size decreases.



Conversely, if the magnitude of the imposed external flow is not sufficient, or if the duration of the flow is too short, the protocol can be unsuccessful in suppressing the spheroid. The spheroid may only have a slower growth rate (Fig. 2(e)) or shrink significantly but resume growing as soon as the external flow is turned off (Fig. 2(f)). Note that for any spheroid size, there exists a critical flow magnitude such that imposing a steady flow at magnitude will eventually lead to the spheroid suppression, while will be unsuccessful. Figure 2(b) shows a protocol with .
In the examples above we have focused on the case where an external volumetric flow is imposed. The same analysis and similar procedures can be used in the case where an external current is imposed (see Fig. 3). We show in Fig. 3(b) a protocol that leads to the suppression of the spheroid. Note that an increased shrinking is obtained if one applies simultaneously an electric current and an external flow, as both effects are additive. Such intervention is displayed in Fig. 3(c) where we observe a faster shrinking due to external flow in addition to the application of an electric current.
Importantly, we emphasize that the protocols we are discussing here are slow and take place on long time scales: using parameters values displayed in Table 3, the examples shown in Figs. 2 and 3 correspond to cm and days. This shows that the suppression of the spheroid requires a slow and steady flow or the application of a small electric current for several weeks.



III.2 Spheroid dynamics with external flows and currents
We now discuss quantitatively how a drain can be used to control the size of a spheroid. To keep the discussion as simple as possible, we consider here the limit of a spheroid enclosing a small lumen (or lumenless). In the limit of a small lumen compared to spheroid size , the differential equation that describes the dynamics of the spheroid shrinks to (see App. B):
| (13) |
where we use the dimensionless radius and time introduced in the previous section. The dimensionless parameters describe the effects of an externally-imposed flow or electric current. If they are set to zero, Eq. (13) reduces to the dynamics of a lumenless spheroid without drain as studied in Ref. Duclut et al. (2019). They are defined as with and where and are the dimensionless volumetric flow and electric current, respectively, as defined above. The effects of an imposed external flow are captured by the coefficients:
| (14) |
Here, describes a permeation effect due to the finite permeability of the spheroid to fluid flows and involves the ratio of the surface permeability and of the (effective) bulk permeability . The parameter includes direct effects of the external flow – proportional to in the dimensional equation corresponding to – which reflects the spheroid volume change imposed by the external flow due to tissue incompressibility (see also App. B). We have furthermore defined
| (15) |
which represents the bioelectric and biohydraulic contribution. Indeed, this term is a sum of terms proportional to the parameters , and thus stems from the coupling between the electric field (or the interstitial fluid flow) and the cell polarity that appears in the cell stress.
Imposing an external electric current also contributes to the spheroid growth control via the parameters:
| (16) |
The coefficient corresponds to electroosmotic flow due to the imposed current. The coefficient accounts for bioelectric and biohydraulic contributions where
| (17) |
Note that since the coefficients are a sum of phenomenological parameters for which we only have order-of-magnitude estimates, it is difficult to obtain a reliable estimate of their magnitude and even of their sign. However, estimating upper bounds for suggests that these bioelectric and biohydraulic contributions are small compared to the other effects. A further discussion of these contributions requires precise estimates of the bioelectric and biohydraulic couplings that could be obtained from experiments on spheroids in presence of a drain.
In equation (13), we have used the definitions Duclut et al. (2019)
| (18) |
Here, is an an effective pumping coefficient and are apparent tensions defined in App. B. The apparent surface tension is a modification of the tissue surface tension stemming from the flexoelectric term proportional to . This flexoelectric contribution plays a crucial role in lumen nucleation Duclut et al. (2019). In the dynamics of the outer radius of the spheroid, its effect is however minor. Finally, the effective pumping combines the active pumping and an electric contribution due to electroosmosis. The parameters introduced above are summarized for convenience in Table 2, and their corresponding values can be computed using Table 1.
| Parameter | Definition | Equation | Description |
|---|---|---|---|
| below Eq. (10) | effective permeation coefficient | ||
| Eq. (40a) | effective pressure | ||
| Eq. (40b) | apparent surface tension | ||
| Eq. (40b) | apparent surface tension | ||
| Eq. (40c) | effective pumping coefficient | ||
| Eq. (12) | characteristic length | ||
| Eq. (12) | characteristic time | ||
| Eq. (12) | characteristic volumetric flux | ||
| Eq. (12) | characteristic electric current | ||
| Eq. (18) | dimensionless effective pressure | ||
| Eq. (18) | dimensionless effective pumping | ||
| Eq. (18) | dimensionless effective velocity | ||
| below Eq. (13) | dimensionless volumetric flow | ||
| below Eq. (13) | dimensionless electric current | ||
| below Eq. (13) | dimensionless external contributions | ||
| Eqs. (14-15) | / | ||
| Eq. (14) | / | ||
| Eqs. (16-17) | / | ||
| Eq. (16) | / |
III.3 Control of the spheroid dynamics
We use Eq. (13) to discuss how external currents and flows can be used to control the growth and shrinkage of a spheroid. Note that whether the spheroid grows () or shrinks () only depends on the numerator of Eq. (13).
Figure 4 shows phase-space trajectories of the outer radius for spheroids that are able to grow in the absence of a drain. Figure 4(a) displays the case without external flow or current. The following scenarios are possible: the spheroid may be growing with an unstable fixed point at (green curve); alternatively, there might be an additional stable fixed point corresponding to a steady-state, either with finite radius (blue curve), or with vanishing radius (orange curve). Imposing an external flow implies nonzero values of and , which are negative in the case where fluid flows out of the lumen (as depicted in Fig. 1(b)). The case of a nonvanishing value of is displayed in Fig. 4(b): in this case, the trajectories are shifted downward by an amount . In this case, even the the growing spheroid (green curve) has a critical radius below which it shrinks (empty green circle). The stable steady-state (blue curve) has moved to . Figure 4(c) shows the effect of a nonvanishing value of : the slope of the phase-space trajectories is modified. Note that this perturbation favors shrinking of the spheroid: the unstable critical radius below which the spheroid shrinks (empty circles along the axis) is shifted to larger values compared to the case without external perturbation shown in Fig. 4(a).
We can estimate the critical volumetric flow needed to change a growing spheroid of size to a shrinking one. From Eq. (13), we obtain
| (19) |
where the minus sign indicates that is required to induce shrinkage (corresponding to flow out of the drain, see Fig. 1(b)). We obtain a similar expression for the critical electric current that is required to induce shrinkage:
| (20) |
Note that and therefore is positive (corresponding to an electric current into the drain, see Fig. 1(a)). One can therefore always find a value of the externally applied flow or current to turn a growing spheroid into a shrinking one.
III.4 State diagrams of hydraulic control of spheroid growth



We conclude this section by presenting state diagrams for a spheroid of initial size to which a steady external flow is imposed, see Fig. 5. We consider a spheroid with a drain inserted to the center but without lumen. This corresponds to choosing equal to the outer radius of the drain. For simplicity, we fix , where is the inner drain radius. We again consider spheroids that can grow in the absence of a drain. Figure 5(a) displays the state diagram for the case where the unperturbed spheroid is growing at all radii (corresponding to the green curve in Fig. 4(a)). Figure 5(b) shows the state diagram for a spheroid which is growing above a critical radius (corresponding to the yellow curve in Fig. 4(a)). Figure 5(c) is the state diagram for spheroids that, in the absence of a drain, either reach a steady-state radius or grow without bounds (corresponding to the blue curve in Fig. 4(a)).
Three different regions exist in the diagrams shown in Fig. 5: (i) a growth region (green), where the imposed flow is not sufficient to arrest growth. Note that for increasing values of , growth slows within this region; (ii) a steady-state region (in orange), in which the spheroid reaches a finite size. Within this region a larger imposed flow drives the spheroid to smaller steady-state sizes; (iii) a suppression region (in blue) where the spheroid shrinks until it is suppressed. We define that a spheroid has been suppressed when its size becomes smaller than a cut-off size , with m a typical cell thickness. In the region of spheroid suppression, the time that it takes for the spheroid to be suppressed is indicated by shades of blue. For fixed initial spheroid size, the time for suppression decreases as the magnitude of the imposed flow increases.
The solid black lines indicate boundaries between the different regions. Note that the line separating the steady-state region and the suppression region depends on the cut-off . The dashed white line indicates the nullcline and gives the critical value of the external flow required to switch from a growing to a shrinking spheroid (given by Eq. (19) for ). Note that below the nullcline the spheroids grow and above the nullcline they shrink. Note that a similar analysis can be performed in the case of an external current . In this case the same behaviors are found: growth, suppression and steady-state regions which depends on the initial spheroid size and on the magnitude of the imposed electric current.
IV Conclusion
Using a coarse-grained description of a tissue as an active material capable of exerting mechanical stresses, transporting ions and pumping fluid, we have proposed a technique to perturb tissues electrically and hydraulically. This technique relies on an imposed fluid flow or electric current source using a micrometric drain or electrode which can turn a growing spheroid to shrinkage.
Our theory allows us to estimate orders of magnitude of the external flow and current that are required to suppress an initially growing spheroid. For small spheroids with radius of about a hundred micrometers, we show for instance that an electric current of a few nanoamperes or a volumetric flow of about m3/s could have a significant impact on the spheroid state (see Fig. 6 in App. E). It means that even a passive drain connecting the inner part of the spheroid to the surrounding medium could already alter growth of small spheroids. For larger aggregates, connecting the drain to a pump leads to spheroid suppression for any size, provided that the imposed external flow is sufficiently strong and maintained long enough (for instance, for spheroids of a few millimeters in radius an electric current of tens to hundreds of nanoamperes or a volumetric flow of about m3/s could be sufficient, see Fig. 6). Spheroid suppression can be obtained also by imposing an electric current, and both fluid flow and electric current application could be combined to accelerate the process.
Our approach has also allowed us to characterize the different long-time states of a growing spheroid subject to an external perturbation. Depending on the magnitude of the hydraulic or electrical field, we have shown that the growth can be either slowed down or arrested (Fig. 5). In the latter case, the arrest of growth can lead to a steady state, or even to the shrinking and eventually the suppression of the spheroid. If the magnitude of the external field is above a threshold value, the suppression of the spheroid is always achieved, and stronger magnitudes then accelerate the process. Our coarse-grained description is generic, as it depends on effective tissue parameters but is insensitive to many details. Our approach does not distinguish between a suppressed state where all cells have disappeared and a state where a few cells still remain. This distinction depends on cellular details that we are not considering here.
We have also shown how different protocols can be used to obtain this suppression: for instance, a longer perturbation at a weaker magnitude is slower but as efficient as a shorter but stronger one; decreasing the magnitude of the flow or electric current as the size of the spheroid decreases can also be used to suppress a spheroid (see Fig. 2 for examples). Interestingly, protocols that could lead to spheroid suppression need to be carried out over a sufficiently long time period. We estimate that the slow, steady flow or electric current that mediates the progressive suppression of a spheroid has to be maintained over days or weeks. This is in contrast with typical cancer ablation techniques Knavel and Brace (2013), for which treatments are brief and intense. Radiofrequency ablation for instance requires the application of an alternating current with high frequency (up to 500 kHz) and high voltage (up to several kV) in order to heat the tissue Erez and Shitzer (1980). Similarly, irreversible electroporation ablations use microsecond pulses of high electric potential (up to 3 kV) Miller et al. (2005).
At the time scales considered in this manuscript, the case of a tumor is more complex than that of a spheroid. First, one has to compare the effect of the flux on the tumor with that on the surrounding healthy tissue. This question will be addressed in future work. Second, we have not explicitly considered the role of nutrient and oxygen transport. The larger division rate at the surface that we consider (see Eq. (9)) could be related for example to oxygen gradient that has been observed experimentally Hirschhaeuser et al. (2010). Furthermore, nutrient transport has been shown to influence growth in other contexts Wang et al. (2017). Moreover, the geometry of spheroids could be more complex than a simple sphere, and a natural expansion of this current work will be the study of the stability of the spherical shape during the growth or shrinkage processes Martin and Risler (2021). Last, during the suppression process, the escape of metastatic cells should be prevented. The signs of stresses that we find here corresponds to flows towards the sphere center. This suggests that escape of material might be hampered by imposed flows or currents, thereby providing a barrier against the escape of cells.
Our work is based on generic mechanical, hydraulic and electrical properties of tissues. It thus paves the way for novel experimental methods to control spheroid size. If applied to cancerous tissues, it could provide a means to influence cancerous tumor growth, arrest their proliferation and even suppress these tumors. Indeed, the hydraulic and electrical perturbations we have proposed here do not rely on identifying and hindering specific chemical pathways that are characteristic of cancerous tissue Hanahan and Weinberg (2011), but rather on general, physical responses to an external field.
Acknowledgments
We thank C.T. Lim and T.B. Saw for useful discussions.
Appendix A Tissues as active two-component fluids
A.1 Mass conservation in growing tissues
In this work, we describe the tissue as a two-component system with (i) a cell phase that accounts for cells and the surrounding extra-cellular matrix, and (ii) the interstitial fluid that permeates the cell phase. Such a description has been introduced in Ref. Ranft et al. (2012). Here, we briefly review this approach to clarify the conceptual basis of Eq. (6) in the main text.
The cell phase is characterized by a cell mass , a cell number density , and a cell volume , and we introduce similarly , and for the interstitial fluid. The assumption that the cells and the fluid fill completely the space is written as:
| (21) |
We use this assumption to define the cell volume fraction and the fluid volume fraction . Mass conservation in the tissue reads:
| (22) |
where is the tissue density and is the total mass flux with the cell and interstitial fluid velocities. As a consequence of cell division and death, cell number is not conserved but obeys a continuity equation:
| (23) |
where and are the rates of cell division and apoptosis respectively. Using mass conservation (22), the cell continuity equation (23), and assuming a constant fluid particle mass, we obtain the continuity equation for the fluid particle density Ranft et al. (2010):
| (24) |
where is the convected time derivative with respect to the cell flow. The above equation implies that a cell of mass can be converted into fluid particles and vice versa when cells die or divide.
We define the total volume flux . Using Eqs. (21), (23) and (24) we obtain the following expression for its divergence Ranft et al. (2012):
| (25) |
where we have defined and the cell and fluid particle mass densities. Assuming that cell and fluid densities are constant and that , the previous equation simplifies to yield Ranft et al. (2012). In the presence of the drain, which imposes a nonvanishing fluid velocity at the inner boundary of the spheroid, the integration of the total flow incompressibility in spherical coordinates yields Eq. (6) in the main text.
A.2 Constitutive equations for the isotropic and anisotropic parts of the cell stress
Following Refs. Ranft et al. (2010, 2012); Sarkar et al. (2019), we derive the constitutive equations for the cell stress for a permeated tissue in the presence of electric fields. We decompose the cell stress tensor into an isotropic contribution and a traceless part , such that .
We first discuss the isotropic cell stress. Cell volume and cell volume fraction are in general functions of the isotropic cell stress , the anisotropic cell stress , the electric field , and the velocity difference . A general equation of state for the cell volume can therefore be written as:
| (26) |
and a similar expression for the cell volume fraction . Since , we can use the equation of state (26) to write the time dependence of the cell number density:
| (27) |
where and , denotes the isotropic and anisotropic compressibilities of the cells, and where we have defined the other compressibility coefficients as , and . To write the constitutive equation for the isotropic stress in a closed form, we now eliminate . For this purpose the cell continuity equation (23) can be rewritten as:
| (28) |
and we specify a constitutive equation for the net growth rate of the tissue . This growth rate in general depends on the isotropic stress and may also depend on , and . In the absence of anisotropic stresses, electric fields and flows, a constant cell density is achieved when cell death compensates cell division. The resulting isotropic cell pressure is the homeostatic pressure . To linear order, the net growth rate near the homeostatic pressure reads Ranft et al. (2012); Sarkar et al. (2019):
| (29) |
where is a constant and will be identified as the bulk viscosity in the following, is a dimensionless coefficient that takes into account the possible dependence of the growth rate on the anisotropic part of the stress, characterizes the influence of the electric field on the growth rate and is a coefficient accounting for the effects of the relative motion of the cells and the interstitial fluid to the growth rate. Using Eqs. (27)-(29), we obtain a general constitutive equation for the isotropic cell stress:
| (30) | ||||
where is and are the isotropic and anisotropic relaxation rates, the relaxation rate associated with the electric field, the relaxation rate arising from a velocity difference. At long times that we consider in the main text, we neglect the relaxation processes and Eq. (30) reduces to
| (31) |
which is Eq. (2a) in the main text. In this long-time limit, cells are described as an active viscous fluid where is revealed as an effective bulk viscosity due to cell division and death.
We now discuss the anisoptropic part of the cell stress . We assume that the tissue behaves as an isotropic elastic material in the absence of cell division and apoptosis. When these events are considered, a reference state for the stress cannot be defined and we therefore express the changes of stress as a differential equation Ranft et al. (2012):
| (32) |
where refers to the corotational time derivative with respect to the cell flow with the cell flow vorticity. This corotational derivative allows us to define a constitutive equation which does not depend on a frame of reference. In general, this term can depend on any of the traceless symmetric tensors in our model, such that at linear order we write:
| (33) |
where characterizes the anisotropic stress relaxation time. The constitutive equation for the anisotropic stress finally reads Ranft et al. (2010); Delarue et al. (2014):
| (34) |
where we have defined the effective tissue shear viscosity . In the main text we consider the long-time limit and the previous equation becomes:
| (35) |
which is Eq. (2b) in the main text.
A.3 Constitutive equations for permeation and for the electric current density
Based on the symmetry of the system and assuming linear response, the constitutive equation for the internal force density reads:
| (36) | ||||
Note that the term vanishes as the polarity is a unit vector. Moreover, in the main text we consider a system with spherical symmetry and the polarity is assumed to be radial , such that the nematic order parameter is diagonal in spherical coordinates with , , . As a consequence, and since for , we obtain Eq. (3) in the main text with , , , and .
Appendix B Dynamics of a spheroid in the presence of a drain
The dynamical equations for the spheroid and lumen radii are obtained by first considering force balance (1) in spherical coordinates with boundary conditions (9). This yields the velocity profiles of cells and interstitial fluid. A differential equation for the radii dynamics is then obtained from the normal stress balance (7) together with the permeation conditions at the boundary given by Eq. (8). Details on this computation in the case without a drain can be found in Ref. Duclut et al. (2019). Including the external fields due to the presence of the drain, we obtain the following coupled differential equations for the dynamics of the radii and of the lumen and of the spheroid:
| (38a) | ||||
| (38b) | ||||
where we have introduced dimensionless radii with and a dimensionless time with . We have also introduced the dimensionless parameters:
| (39) |
where the effective parameters are defined as:
| (40a) | |||
| (40b) | |||
| (40c) | |||
In addition to these terms, the presence of external flows and currents gives new dimensionless contributions which are defined as:
| (41a) | |||
| (41b) | |||
| (41c) | |||
| (41d) | |||
where is an effective permeation coefficient.
To understand the effect of an external flux and an external electric current on the spheroid size, we can rewrite Eq. (38b) in the form of an equation for the dynamics of the spheroid volume . To simplify the analysis, we take a vanishing lumen size () and we consider only the terms that comes from the externally imposed current and flux. It yields:
| (42) |
where we have reintroduced dimensional quantities to simplify the following discussion. The first term on the right-hand side of this equation is a direct consequence of imposing an external flow: since the tissue is incompressible, an imposed flow from the outside to the inside of the spheroid () provokes an increase in volume, while an imposed flow from the inside of the spheroid to the outside () shrinks its size. The second term is a signature of the finite permeability of the tissue, which acts as a porous medium, and the effect is therefore proportional to the ratio of the surface permeability and the bulk permeability . The third term accounts for a similar phenomenon as the second one but due to the electroosmotically generated flow due to the imposed current. The two last terms correspond to bioelectric and biohydraulic contributions: indeed, the terms and involve the parameters and thus stem from the coupling between the electric field (or the interstitial fluid flow) and the cell polarity that appears in the cell stress.
In the case of a small lumen compared to the spheroid size, one can consider the limit to describe the spheroid dynamics. In this limit, we can in particular obtain from Eq. (38b) an equation for the dynamics of only, which is given by Eq. (13) in the main text. The parameters introduced in the main text read and .
Appendix C Imposed pressure and electric potential difference
The main text describes the spheroid size control when an external volumetric flow or an external current is imposed. Alternatively, a pressure difference or an electric potential difference can be imposed between the outer part of the drain and the outer layer of the spheroid, which can be considered as the conjugate ensemble. In this appendix, we provide the additional equations required to discuss this situation.
C.1 Imposed pressure difference
In order to compute the pressure difference , we decompose it as where is the pressure difference across the spheroid and is the pressure difference between the outer part of the drain and the lumen and reads:
| (43) |
where are the hydraulic resistances of the drain and of the lumen, respectively. The drain hydraulic resistance is given by , where mPas is the viscosity of the interstitial fluid, the width and radius of the drain.
The pressure difference can then be computed using Eq. (8). In dimensionless form, it reads:
| (44) |
where we use the short-hand notation and we have defined:
| (45) | ||||
The dimensionless quantities , , and have been introduced in App. B. To compute the interstitial pressure difference , one can use the identity . Indeed, the gradient of the interstitial fluid pressure can be obtained using force balance (1b) and the constitutive equations (3) and (4). It reads:
| (46) |
where we have introduced, in addition to the dimensionless quantities already introduced in App. B, the dimensionless radius , cell velocity , and interstitial fluid pressure and the dimensionless parameter . The dimensionless cell velocity appearing in Eq. (46) can be cast into the following form:
| (47) |
where we have introduced the following -independent factors:
| (48a) | ||||
| (48b) | ||||
| (48c) | ||||
and the following dimensionless quantities (in addition to those already introduced in App. B):
| (49) |
The dimensionless pressure difference (44) can therefore be computed in terms of the dimensionless parameters of the model, although its expression is lengthy. One can however expand this expression in the limit of a thin spheroid (i.e ), yielding the expression:
| (50) |
where the first term on the right-hand side is the Laplace contribution in the limit and the dimensionless coefficient represents the first correction in term of the thickness and reads:
| (51) | ||||
C.2 Imposed electric potential difference
Similarly to the pressure, one can compute the electric potential difference by decomposing it as with the electric potential difference across the spheroid and with the electric potential difference across the drain. This difference reads
| (52) |
where are the electrical conductances of the drain and of the lumen. The electrical conductance of the drain is computed as , where m is the resistivity of the interstitial fluid (taken to be that of salted water, whose conductivity is about S/m).
The electric potential difference across the spheroid can then be computed in the following way. From Eq. (4), an expression for the electric field can be obtained, which can then be used to compute the electric potential difference using the identity . Defining the dimensionless electric field , we have:
| (53) |
where we have defined the dimensionless parameters:
| (54) |
and where the expression for the dimensionless cell velocity is displayed in Eq. (47).


Appendix D Numerical solution to the dynamics equations
Figures 2 and 3 of the main text have been obtained by solving the coupled equations (38a) and (38b) using the dimensionless parameters given in Table 3. The presence of the drain provides a natural cut-off for the (dimensionless) lumen radius : if at a time the inner radius reaches the size of the outer drain radius, which we assume to be equal to m, we fix its (dimensionless) value to be and solve only Eq. (38b) with this fixed value for . If the external flow is stopped at a subsequent time (see Fig. 2(f) of the main text for instance), we restart solving simultaneously Eqs. (38a) and (38b), taking as the initial condition.
Figure 5 of the main text has been obtained by taking and solving numerically equation (38b) using the dimensionless parameters given in Table 3, with the initial condition and a given imposed external flux . If a spheroid reaches a cut-off size after a time , it is classified as “suppressed” and its suppression time is used to produce the color coding.
Appendix E Estimates for parameter values
Estimation of the phenomenological parameters appearing in the continuum model is crucial for our analysis. Some of these parameters, such as the cell shear and bulk viscosities, the tissue surface tension and the bulk permeability for instance, have already been estimated in experiments. For most of the remaining parameters, experimental values are not yet available and we have used order-of-magnitude estimations (see Refs. Sarkar et al. (2019) and Duclut et al. (2019) for details). We provide parameters values and references for these values in Table 1.
| Figure | Parameters values | |||||||||||||
| 2 | 0.1 | -1 | 1 | 0 | -0.05 | 0.15 | 0 | 1 | 0.1 | 0 | 0 | 0 | 0 | |
| 3 | 0.3 | -1 | 1 | 0 | 0.15 | 0.15 | 0.2 | 0.1 | 0.5 | 0 | 0 | 0 | 0 | |
| 5(a) | - | -1 | 1 | 0 | - | 0.15 | 0.5 | - | 0.3 | 0 | 0 | 0 | 0 | |
| 5(b) | - | 1 | 1 | -0.15 | - | 0.15 | 5.5 | - | 0 | 0 | 0 | 0 | 0 | |
| 5(c) | - | 1 | 1 | 0 | - | 0.15 | 4 | - | 0.15 | 0 | 0 | 0 | 0 | |
References
- Green and Sharpe (2015) J. B. A. Green and J. Sharpe, “Positional information and reaction-diffusion: Two big ideas in developmental biology combine,” Development 142, 1203–1211 (2015).
- Miller and Davidson (2013) C. J. Miller and L. A. Davidson, “The interplay between cell signalling and mechanics in developmental processes,” Nat. Rev. Genet. 14, 733–744 (2013).
- Mammoto et al. (2013) T. Mammoto, A. Mammoto, and D. E. Ingber, “Mechanobiology and Developmental Control,” Annu. Rev. Cell Dev. Biol. 29, 27–61 (2013).
- Ladoux and Mège (2017) B. Ladoux and R.-M. Mège, “Mechanobiology of collective cell behaviours,” Nat. Rev. Mol. Cell Biol. 18, 743–757 (2017).
- Ruiz-Herrero et al. (2017) T. Ruiz-Herrero, K. Alessandri, B. V. Gurchenkov, P. Nassoy, and L. Mahadevan, “Organ size control via hydraulically gated oscillations,” Development 144, 4422–4427 (2017).
- Dumortier et al. (2019) J. G. Dumortier, M. Le Verge-Serandour, A. F. Tortorelli, A. Mielke, L. de Plater, H. Turlier, and J.-L. Maître, “Hydraulic fracturing and active coarsening position the lumen of the mouse blastocyst,” Science 365, 465–468 (2019).
- Levin and Martyniuk (2018) M. Levin and C. J. Martyniuk, “The bioelectric code: An ancient computational medium for dynamic control of growth and form,” Biosystems 164, 76–93 (2018).
- Silver and Nelson (2018) B. B. Silver and C. M. Nelson, “The Bioelectric Code: Reprogramming Cancer and Aging From the Interface of Mechanical and Chemical Microenvironments,” Front. Cell Dev. Biol. 6, 21 (2018).
- Chan et al. (2019) C. J. Chan, M. Costanzo, T. Ruiz-Herrero, G. Mönke, R. J. Petrie, M. Bergert, A. Diz-Muñoz, L. Mahadevan, and T. Hiiragi, “Hydraulic control of mammalian embryo size and cell fate,” Nature 571, 112–116 (2019).
- Fütterer et al. (2003) C. Fütterer, C. Colombo, F. Jülicher, and A. Ott, “Morphogenetic oscillations during symmetry breaking of regenerating Hydra vulgaris cells,” EPL 64, 137–143 (2003).
- Roux (1892) W. Roux, “über die morphologische Polarisation von Eiern und Embryonen durch den electrischen Strom,” Math. Naturwiss. Kl. 101, 27–228 (1892).
- McCaig et al. (2005) C. D. McCaig, A. M. Rajnicek, B. Song, and M. Zhao, “Controlling Cell Behavior Electrically: Current Views and Future Potential,” Physiol. Rev. 85, 943–978 (2005).
- McLaughlin and Levin (2018) K. A. McLaughlin and M. Levin, “Bioelectric signaling in regeneration: Mechanisms of ionic controls of growth and form,” Dev. Biol. 433, 177–189 (2018).
- Watanabe et al. (2009) H. Watanabe, T. Fujisawa, and T. W. Holstein, “Cnidarians and the evolutionary origin of the nervous system: Cnidarian nervous system,” Develop. Growth Differ. 51, 167–183 (2009).
- Braun and Ori (2019) E. Braun and H. Ori, “Electric-Induced Reversal of Morphogenesis in Hydra,” Biophys. J. 117, 1514–1523 (2019).
- Martín-Belmonte et al. (2008) F. Martín-Belmonte, W. Yu, A. Rodríguez-Fraticelli, A. Ewald, Z. Werb, M. A. Alonso, and K. Mostov, “Cell-Polarity Dynamics Controls the Mechanism of Lumen Formation in Epithelial Morphogenesis,” Curr. Biol. 18, 507–513 (2008).
- Debnath et al. (2002) J. Debnath, K. R. Mills, N. L. Collins, M. J. Reginato, S. K. Muthuswamy, and J. S. Brugge, “The Role of Apoptosis in Creating and Maintaining Luminal Space within Normal and Oncogene-Expressing Mammary Acini,” Cell 111, 29–40 (2002).
- Huch and Koo (2015) M. Huch and B.-K. Koo, “Modeling mouse and human development using organoid cultures,” Development 142, 3113–3125 (2015).
- Dahl-Jensen and Grapin-Botton (2017) S. Dahl-Jensen and A. Grapin-Botton, “The physics of organoids: A biophysical approach to understanding organogenesis,” Development 144, 946–951 (2017).
- Alcaraz et al. (2008) J. Alcaraz, R. Xu, H. Mori, C. M. Nelson, R. Mroue, V. A. Spencer, D. Brownfield, D. C. Radisky, C. Bustamante, and M. J. Bissell, “Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia,” EMBO J. 27, 2829–2838 (2008).
- Montel et al. (2011) F. Montel, M. Delarue, J. Elgeti, L. Malaquin, M. Basan, T. Risler, B. Cabane, D. Vignjevic, J. Prost, G. Cappello, and J.-F. Joanny, “Stress Clamp Experiments on Multicellular Tumor Spheroids,” Phys. Rev. Lett. 107, 188102 (2011).
- Delarue et al. (2013) M. Delarue, F. Montel, O. Caen, J. Elgeti, J.-M. Siaugue, D. Vignjevic, J. Prost, J.-F. Joanny, and G. Cappello, “Mechanical Control of Cell flow in Multicellular Spheroids,” Phys. Rev. Lett. 110, 138103 (2013).
- Kruse et al. (2005) K. Kruse, J. F. Joanny, F. Jülicher, J. Prost, and K. Sekimoto, “Generic theory of active polar gels: A paradigm for cytoskeletal dynamics,” Eur. Phys. J. E 16, 5–16 (2005).
- Marchetti et al. (2013) M. C. Marchetti, J. F. Joanny, S. Ramaswamy, T. B. Liverpool, J. Prost, M. Rao, and R. A. Simha, “Hydrodynamics of soft active matter,” Rev. Mod. Phys. 85, 1143–1189 (2013).
- Ranft et al. (2010) J. Ranft, M. Basan, J. Elgeti, J.-F. Joanny, J. Prost, and F. Jülicher, “Fluidization of tissues by cell division and apoptosis,” Proc. Natl. Acad. Sci. U.S.A. 107, 20863–20868 (2010).
- Sarkar et al. (2019) N. Sarkar, J. Prost, and F. Jülicher, “Field induced cell proliferation and death in a model epithelium,” New J. Phys. 21, 043035 (2019).
- Duclut et al. (2019) C. Duclut, N. Sarkar, J. Prost, and F. Jülicher, “Fluid pumping and active flexoelectricity can promote lumen nucleation in cell assemblies,” Proc. Natl. Acad. Sci. U.S.A. 116, 19264–19273 (2019).
- Scott et al. (2012) A. M. Scott, J. D. Wolchok, and L. J. Old, “Antibody therapy of cancer,” Nat. Rev. Cancer 12, 278–287 (2012).
- Baskar et al. (2012) R. Baskar, K. A. Lee, R. Yeo, and K.-W. Yeoh, “Cancer and Radiation Therapy: Current Advances and Future Directions,” Int. J. Med. Sci. 9, 193–199 (2012).
- Al-Bataineh et al. (2012) O. Al-Bataineh, J. Jenne, and P. Huber, “Clinical and future applications of high intensity focused ultrasound in cancer,” Cancer Treat. Rev. 38, 346–353 (2012).
- Mittelstein et al. (2020) D. R. Mittelstein, J. Ye, E. F. Schibber, A. Roychoudhury, L. T. Martinez, M. H. Fekrazad, M. Ortiz, P. P. Lee, M. G. Shapiro, and M. Gharib, “Selective ablation of cancer cells with low intensity pulsed ultrasound,” Appl. Phys. Lett. 116, 013701 (2020).
- Tijore et al. (2020) A. Tijore, F. Margadant, M. Yao, A. Hariharan, C. A. Z. Chew, S. Powell, G. K. Bonney, and M. Sheetz, “Ultrasound-mediated mechanical forces selectively kill tumor cells,” bioRxiv 10.1101, 2020.10.09.332726 (2020).
- Miller et al. (2005) L. Miller, J. Leor, and B. Rubinsky, “Cancer Cells Ablation with Irreversible Electroporation,” Technol. Cancer Res. Treat. 4, 699–705 (2005).
- Perkons et al. (2018) N. R. Perkons, E. J. Stein, C. Nwaezeapu, J. C. Wildenberg, K. Saleh, R. Itkin-Ofer, D. Ackerman, M. C. Soulen, S. J. Hunt, G. J. Nadolski, and T. P. Gade, “Electrolytic ablation enables cancer cell targeting through pH modulation,” Commun. Biol. 1, 48 (2018).
- Knavel and Brace (2013) E. M. Knavel and C. L. Brace, “Tumor Ablation: Common Modalities and General Practices,” Tech. Vasc. Interv. Radiol. 16, 192–200 (2013).
- Ranft et al. (2012) J. Ranft, J. Prost, F. Jülicher, and J.-F. Joanny, “Tissue dynamics with permeation,” Eur. Phys. J. E 35, 46 (2012).
- Dolega et al. (2017) M. E. Dolega, M. Delarue, F. Ingremeau, J. Prost, A. Delon, and G. Cappello, “Cell-like pressure sensors reveal increase of mechanical stress towards the core of multicellular spheroids under compression,” Nat. Commun. 8, 14056 (2017).
- Basan et al. (2009) M. Basan, T. Risler, J.-F. Joanny, X. Sastre-Garau, and J. Prost, “Homeostatic competition drives tumor growth and metastasis nucleation,” HFSP J. 3, 265–272 (2009).
- Simha and Ramaswamy (2002) R. Simha and S. Ramaswamy, “Hydrodynamic Fluctuations and Instabilities in Ordered Suspensions of Self-Propelled Particles,” Phys. Rev. Lett. 89, 058101 (2002).
- Bittig et al. (2008) T. Bittig, O. Wartlick, A. Kicheva, M. González-Gaitán, and F. Jülicher, “Dynamics of anisotropic tissue growth,” New J. Phys. 10, 063001 (2008).
- Blackiston et al. (2009) D. J. Blackiston, K. A. McLaughlin, and M. Levin, “Bioelectric controls of cell proliferation: Ion channels, membrane voltage and the cell cycle,” Cell Cycle 8, 3527–3536 (2009).
- Darcy (1856) H. P. G. Darcy, Les Fontaines Publiques de La Ville de Dijon (Dalmont, Paris, France, 1856).
- Ramaswamy et al. (2000) S. Ramaswamy, J. Toner, and J. Prost, “Nonequilibrium Fluctuations, Traveling Waves, and Instabilities in Active Membranes,” Phys. Rev. Lett. 84, 3494–3497 (2000).
- Kirby (2013) B. J. Kirby, Micro- and Nanoscale Fluid Mechanics (Cambridge University Press, Cambridge, England, 2013).
- Delarue et al. (2014) M. Delarue, J.-F. Joanny, F. Jülicher, and J. Prost, “Stress distributions and cell flows in a growing cell aggregate,” Interface Focus 4, 20140033 (2014).
- Forgacs et al. (1998) G. Forgacs, R. A. Foty, Y. Shafrir, and M. S. Steinberg, “Viscoelastic Properties of Living Embryonic Tissues: A Quantitative Study,” Biophys. J. 74, 2227–2234 (1998).
- Netti et al. (2000) P. A. Netti, D. A. Berk, M. A. Swartz, A. J. Grodzinsky, and R. K. Jain, “Role of Extracellular Matrix Assembly in Interstitial Transport in Solid Tumors,” Cancer Res. 60, 2497 (2000).
- Brace and Guyton (1977) R. A. Brace and A. C. Guyton, “Interaction of transcapillary Starling forces in the isolated dog forelimb,” Am. J. Physiol. 233, H136–H140 (1977).
- Erez and Shitzer (1980) A. Erez and A. Shitzer, “Controlled Destruction and Temperature Distributions in Biological Tissues Subjected to Monoactive Electrocoagulation,” J. Biomech. Eng. 102, 42–49 (1980).
- Hirschhaeuser et al. (2010) F. Hirschhaeuser, H. Menne, C. Dittfeld, J. West, W. Mueller-Klieser, and L. A. Kunz-Schughart, “Multicellular tumor spheroids: An underestimated tool is catching up again,” J. Biotechnol. 148, 3–15 (2010).
- Wang et al. (2017) X. Wang, H. A. Stone, and R. Golestanian, “Shape of the growing front of biofilms,” New J. Phys. 19, 125007 (2017).
- Martin and Risler (2021) M. Martin and T. Risler, “Viscocapillary instability in cellular spheroids,” New J. Phys. 23, 033032 (2021).
- Hanahan and Weinberg (2011) D. Hanahan and R. A. Weinberg, “Hallmarks of Cancer: The Next Generation,” Cell 144, 646–674 (2011).