Flexible String Model of Unsaturated Lipid Bilayer
Abstract
An analytically solvable model of unsaturated lipid bilayer is derived by introducing finite bending angle of the unsaturated bond relative to straight part of the lipid chain considered previously in our model of semi-flexible strings. It is found that lateral pressure profile of unsaturated lipids has distinct maximum in the unsaturated bond region due to enhanced excluded volume effect caused by the bent bond, leading to an increase of entropic repulsion between the lipid chains. Simultaneously, just away from the unsaturated bond some parts of the neighbouring lipid chains have less probability to collide by geometrical reasons, causing depletion of entropic repulsion relative to saturated lipid chains case and resulting in the local minima of the lateral pressure profile surrounding the maximum at the bent unsaturated bond. Lipid chain order parameter, lateral pressure profile, and area per lipid are computed for POPC and compared with those for DPPC lipid bilayer.
I Introduction
Consider lipid bilayers that differ only by lipid chain unsaturation: one bilayer consists of saturated lipids, and the other one of unsaturated lipids. Compared to saturated lipid bilayer, unsaturated one would have larger area per lipid (at the same temperature) [1, 2], lower gel phase transition temperature [1], a drop in the chains order parameter [3, 4], and a different lateral pressure profile [3]. In this paper we reproduce all these characteristic features by making minimal, but pivotal changes to our previously developed analytically solvable flexible string model [5] of saturated lipid bilayer. The changes are two. The principal one is made in the self-consistent calculation procedure: lipid unsaturation is introduced by allowing only bent chain conformations at the double bond position, while the others are filtered out from the summation over lipid chain conformations in the partition function of the lipid bilayer. The second change lies in a slight () tuning of the flexible-string model parameter for incompressible chain’s cross-section area of unsaturated lipid relative to saturated one. The latter was done to match equilibrium area per lipid for the unsaturated bilayer found in [1, 2].
For reference we used DPPC (16:0) lipids for saturated bilayer, and POPC (18:1/16:0) lipids for unsaturated bilayer. These two cases were chosen as ones of the most well-characterized lipids. POPC is monounsaturated lipid; poly-unsaturated lipids can also be modelled within our model approach, however here we have concentrated solely on DPPC-POPC bilayer differences.
We start with a short overview of the flexible string model for saturated lipid bilayer, including liquid-gel phase transition visible in a free energy plot. These calculations mostly reproduce our previous work [6] with minor simplifications. We then introduce lipid chains unsaturation via specially developed conformational filtering procedure in partition function of the lipids system, which is followed by derivations of analytical expressions for the order parameter and lateral pressure profile. Finally, we apply these approaches to DPPC and POPC models and discuss the results.
I.1 Flexible String Model of Saturated Lipid
In dynamic mean-field approach, lipid bilayer can be modelled as a single lipid exposed to the self-consistently derived potential, see Fig. 1. Mean-field parameters being derived from the chosen bilayer composition and environment parameters.


For a concrete approach we use a model of a single lipid in the mean-field potential that arises from entropic repulsion between fluctuating neighbouring lipid chains due to excluded volume effect [5]. The minimal model consists of a flexible string in a parabolic mean-field potential, see Fig. 2. The energy functional of the string consists of kinetic energy, bending energy, and energy arising from the confining mean-field potential:
| (1) |
here is a vector in the plane of the membrane giving deviation of a string from the axis (see Fig. 2), is a string linear density, is a string bending rigidity, and is self-consistently derived parameter of entropic repulsion strength, that characterises the parabolic mean-field potential as function of a deviation of the string centres from -axis, , is a hydrophobic thickness of the monolayer.
Let’s find expression for the free energy with the energy functional , Eq. 1. For this, we impose boundary conditions on the , this in turn allows us to represent in the operator form, and finally express free energy in terms of eigenvalues of that operator.
Boundary conditions for a model flexible string take into account the following physical assumptions (same is assumed also for component ):
| the monolayer normal at membrane surface; | |||
| (2) | |||
The first boundary condition reflects the orientational asymmetry of the monolayer due to the water-lipid interface which is clearly seen from data on the molecules orientational order parameter [7, 8]: lipid tails are more ordered in the vicinity of head-groups constrained by the hydrophobic tension. Yet, the tilt transition when director forms finite angle with the monolayer normal is not considered here [kheyfets_tilt_2019]. The other boundary conditions reflect the freely moving lipid head-group and hydrocarbon tail end: zero force acting on the head-group and zero torque and force acting on the lipid tail end.
Under the boundary conditions Eq. (I.1) potential energy part of the functional Eq. (1) can be equivalently rewritten in terms of linear Hermitian operator in the form:
| (3) | ||||
Then, an arbitrary conformation of the chain can be expressed as the deviation of the centers of the string, , from the straight vertical line (see Fig. 2), and is parameterized by a set of coefficients of the linear decomposition of the function over the eigenfunctions of the operator :
| (4) | ||||
Number of coefficients is related to the number of groups in the single chain of the lipid.
Substituting Eq. (4) into Eq. (1) and using the standard orthogonality property of the eigenfunctions of operator , enables a simple decomposition of the energy functional into the series:
| (5) |
Hence, Eq. (5) reduces the problem of calculating the free energy of the spring to a sum of free energies of non-interecting 1-dimensional harmonic oscillators with ”spring rigidities” and ”coordinates” . The corresponding eigenvalues and orthonormalized eigenfunctions of the operator are [5]:
| (6) | ||||||
Arbitrary chain conformation is thus : . Due to normalization, dimensionality is , and hence dimensionality of . Assuming membrane to be locally isotropic in the lateral plane, we split partition function into a product of two equal components, , and thus free energy of the lateral oscillations of the chain equals to [9]:
| (7) |
In order to see how depends on the area per lipid, (see Fig. 2), we note that expression can be computed in two ways - directly via Eqs.(6,7):
| (8) |
and also by representing partition function, as path integral over all chain conformations, :
| (9) |
here denotes path integration. Hence, Eq.(I.1) shows that is proportional to the mean area swept by the centers of the chain. The latter area is also related to the mean area swept by the chain, , and incompressible area of the chain, , as: . Hence we conclude that:
| (10) |
| (11) |
We conclude that is a monotonically decreasing function of , and hence is also monotonically decreasing function of (see Eqs. (6,7)). Eq.(11) constitutes self-consistency equation as it relates mean-field parameter, , with the area per lipid, .
Another contribution to the string’s free energy is surface energy, , where is a hydrophobic tension of the lipid membrane, and is mean area swept by the string. Thus, is monotonically increasing function of mean area swept by the chain. has a single minimum as a function of , while two minima are needed in order to reproduce liquid to gel phase transition. Hence, in order to get liquid-gel first-order phase transition we need to add one more contribution to the free energy. That contribution is van der Waals interaction between the neighbouring lipids.
Analytical expression for attractive van der Waals interaction between two hydrocarbon chains has been calculated in [10] on the basis of quantum mechanics:
| (12) |
here is an interaction constant, and is a lipid’s cross-section diameter.
In the equilibritum free energy of the chain, , is minimal, hence we require that
| (13) | ||||
Here we used renormalized van der Waals coefficient which arises from using area per lipid instead of lipid diameter, and additional factor of 3 accounts for the fact that every lipid is surrounded by other lipids, assuming a tightly packed lattice, which has a coordination number 6.
Solving minimum energy condition, Eq.(13), together with self-consistency equation, Eq.(11), for and allows to find equilibrium area per lipid at the free energy minima, see Figs. 3 and 4. We used the following input parameters: incompressible area Å2, Å, erg/cm2, string bending rigidity , kcal Å6/mol, number of groups per chain, as a maximal number of oscillation modes of the string.


II Calculation
II.1 Flexible String Model of Unsaturated Lipid
Let us introduce unsaturated bond in the lipid chain by allowing only bent chain conformations in the partition function of the string. Dropping conformation independent kinetic energy contributions, one finds:
| (14) | |||||
Here Dirac delta function filters out string conformations that do not have a particular bending angle at the depth coordinate inside the bilayer , i.e. at the position of the double bond. , and is tangent angle of the chain at the double bond, takes into account that the chain is bent in either of two sides, i.e. correspondently, with the same probability.
For self-consistency equation, Eq.(10), we can concentrate on the parts of the that include .
| (15) |
where is introduced to keep the corresponding part dimensionless. One has to use the following trick to calculate the above path-integral. Namely, the Dirac delta function is expressed using the well known identity:
| (16) |
Hence, substituting the Dirac delta function in Eq.(II.1) by identity from Eq.(16) and integrating first over all the and then over , one finds:
| (17) |
where the last equality in Eq. (II.1) follows from the fact that depends only on , and hence . Substituting this into Eq.(7) one finds:
| (18) |
Self-consistency equation for unsaturated lipids then reads:
(compare with Eq.(11)). The equilibrium condition is:
| (20) |
(compare with Eq.(13)).
Solving self-consistency condition, Eq.(II.1), together with equilibrium condition, Eq.(20), for and allows to find equilibrium area per lipid, see Fig.5. All the distinct differences with the saturated lipids case in Figs. 3 and 4 are solely due to inclusion of Dirac delta function filter into saturated lipids partition function, Eq.(14), where we have used corresponding to double bond bending angle . Slight decrease of incompressible string area for unsaturated lipids: Å2 instead of saturated lipid case: Å2 was necessary to keep equilibrium area per lipid in the range found earlier [1, 2].

Computed equilibrium areas per lipid for POPC shown on Fig. 5 are slightly above the values reported in the literature [1, 11, 12, 2]. This means that one should change some other parameters of the calculation for the closer match with experimental data on area per lipid. However, in this study we focus on a single most important feature of the unsaturated lipids, namely, the double bond, which is introduced by imposing a filter in the partition function, Eq.(14). In order to see the effect of this particular change, we had chosen to make as little changes as possible to the other calculation parameters.
II.2 Lateral Pressure Profile
Lateral pressure profile is a function which is equal to hydrophobic tension, , when integrated over the thickness of the monolayer:
| (21) |
Comparing this with equilibrium condition, Eq.(13) or Eq.(20), we see that it has two components – one is due to entropic repulsion, , and the other is due to van der Waals attraction, :
| (22) |
For saturated lipids, lateral pressure profile due to entropic repulsion is
| (23) |
By definition, and are eigenvalues and eigenfunctions of operator in Eq. 3: . Hence we write
| (24) | ||||
Comparing this with Eq.(23) we conclude that
| (25) | ||||
Similarly, for unsaturated lipids, one arrives at the following expression for lateral pressure profile:
| (26) |
Lateral pressure profile plots for saturated and unsaturated lipids are plotted in Fig. 6. We see that lateral pressure peak at the midplane area () becomes smaller with unsaturation. Lateral pressure profile was studied in [3] by means of molecular dynamics: in Fig. 7 of their paper [3] the authors plot lateral pressure profile of lipids with increasing unsaturation and also find that the peak at the midplane area diminishes with unsaturation.

II.3 Order Parameter
By definition, molecular order parameter is
| (27) |
In flexible string model we have an approximation for tangent:
| (28) |
hence we express via as:
| (29) |
Also note that
| (30) | ||||
Thus, intermediate expression for the order parameter is
| (31) |
Starting with saturated lipids, we write:
| (32) |
The numerator integral here is:
| (33) |
The denominator integral is the same, but with . Hence, one obtains:
| (34) |
Thus, we see that expression in Eq.(32) is real, hence it coincides with its complex conjugate, so we conclude that Eq.(30) for saturated lipids reads:
| (35) | ||||
where:
| (36) |
and order parameter for saturated lipids is
| (37) |
Similarly, for unsaturated lipids we start with :
| (38) | ||||
Consider the numerator integral first. Using Dirac delta function representation in Eq.(16), completing the square for , integrating over and then again completing the square for and integrating over afterwards one finds:
| (39) | ||||
| (40) | ||||
Mean for complex conjugate, , will be the same, but with changing to in Eq.(40). Sum of these two is real:
| (41) |
where now:
| (42) |
| (43) |
Then, Eq.(30) for unsaturated lipids is:
| (44) |
Calculating this integral we finally arrive at the expression for order parameter of unsaturated lipids:
| (45) |
Order parameter plots for saturated Eq.(37) and unsaturated Eq.(45) lipids are plotted in Fig. 7. The order parameter curve Eq.(45), corresponding to e.g. the case of unsaturated POPC, has a drop in the middle of the monolayer, where double bond is located at in our model. This result coincides with the molecular dynamics study, [3] (see Fig. 10 therein) and [4] (see Fig. 2 therein).
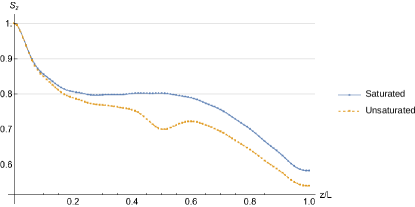
In passing, we note that order parameter for DPPC and POPC has also been reported in [2] (Fig. 13 therein), but the authors had plotted order parameter only for saturated POPC chain, which closely matches order parameter for DPPC.
III Discussion
To summarise, we have modelled unsaturated POPC lipid in our flexible strings model by allowing only strings conformations that possess fixed nonzero tangent angle of the double bond with respect to preceding it saturated bonds at some definite position inside the bilayer, e.g. etc. This results in larger area per lipid compared to saturated lipids, as e.g. in DPPC lipid, and produces lateral pressure profiles and order parameter distributions that match qualitatively the ones reported in [3]. The most important physics conclusion that follows from our results is obvious from Figs. 6, 10. Namely, the lateral pressure profiles of unsaturated lipids near the unsaturated bonds go beyond saturated lipids profile, as would be expected from considerations of excluded volume effect on the entropic repulsion between the lipid chains. The unsaturated bond has finite bending angle with respect to straight line of the lipid chain and, therefore, increases locally the lipid occupied area, thus causing stronger entropic repulsion between the lipid chains. Simultaneously, away from the unsaturated (bent) bond the neighbouring lipid chains have less probability to collide just by geometrical reasons, and therefore, the entropic repulsion between them is depleted relative to saturated lipid chains case, thus causing relative decrease of the lateral pressure profile with respect to saturated lipids case in these same regions away from the bent unsaturated bond, as is seen in Figs. 6, 10.
In support of the above reasoning we also have checked predictions of our model for the cases of unsaturated double bond with different values of the bending angle and with different positions of the unsaturated bond along the lipid chains.
Let’s start with different values of the double bond angle. Presented here Fig. 8 shows lateral pressure profiles for various values of the fixed double bond tangent angle calculated in our model, see Eq. (26). We see that with decreasing double bond tangent angle the lateral pressure profile approaches the one of the saturated lipid, calculated using Eq. (25).

Next, we consider effect of double bond tangent angle on the behaviour of the order parameter for various values of the angle. Results of calculations using Eq. (45)are presented in Fig. 9. We see that for angles less then at the order parameter exceeds the one of the saturated lipid (), see Fig. 7. This means, that average tangent value of an angle of saturated lipid at is about .

Farther, we investigated an influence of the position of the double bond on the membrane characteristics. Lateral pressure profiles calculated for various double bond positions using Eq. (26) are exhibited in Fig. 10. Comparison of the latter with the lateral pressure profile of the saturated lipid in Fig. 6 supports proposed above relation between position of unsaturated bond and local excluded volume effect. Also, we see that in our model the closer double bond is to the headgroup of the lipid, the less is its impact on the shape of the lateral pressure profile. This is clearly due to the fixed angle at the headgroup, , see Eq.(I.1).

Computed order parameter distributions for various values of the double bond position using Eq.(45) are shown in Fig. 11. Comparing the latter with the order parameter distribution of the saturated lipid, Fig. 7, we see that the double bond position perturbs order parameter locally, so that before and after the double bond the order parameter curve gradually converges to the one of the saturated lipid.

IV Acknowledgements
Authors acknowledge support by the Federal Academic Leadership Program Priority 2030 (NUST MISIS Grant No. K2-2022-025).
References
- Kučerka et al. [2011] N. Kučerka, M.-P. Nieh, and J. Katsaras, Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature, Biochimica et Biophysica Acta (BBA) - Biomembranes 1808, 2761 (2011).
- Patra et al. [2006] M. Patra, E. Salonen, E. Terama, I. Vattulainen, R. Faller, B. W. Lee, J. Holopainen, and M. Karttunen, Under the Influence of Alcohol: The Effect of Ethanol and Methanol on Lipid Bilayers, Biophysical Journal 90, 1121 (2006).
- Ollila et al. [2007] S. Ollila, M. T. Hyvönen, and I. Vattulainen, Polyunsaturation in Lipid Membranes: Dynamic Properties and Lateral Pressure Profiles, The Journal of Physical Chemistry B 111, 3139 (2007).
- Pyrkova et al. [2011] D. V. Pyrkova, N. K. Tarasova, T. V. Pyrkov, N. A. Krylov, and R. G. Efremov, Atomic-scale lateral heterogeneity and dynamics of two-component lipid bilayers composed of saturated and unsaturated phosphatidylcholines, Soft Matter 7, 2569 (2011).
- Mukhin and Baoukina [2005] S. I. Mukhin and S. Baoukina, Analytical derivation of thermodynamic characteristics of lipid bilayer from a flexible string model, Physical Review E 71, 061918 (2005).
- Kheyfets et al. [2018] B. Kheyfets, T. Galimzyanov, and S. Mukhin, Microscopic Description of the Thermodynamics of a Lipid Membrane at Liquid-gel Phase Transition, JETP Letters 107, 718 (2018).
- Lindahl and Edholm [2000] E. Lindahl and O. Edholm, Mesoscopic undulations and thickness fluctuations in lipid bilayers from molecular dynamics simulations., Biophysical Journal 79, 426 (2000).
- Vermeer et al. [2007] L. Vermeer, B. de Groot, V. Réat, A. Milon, and J. Czaplicki, Acyl chain order parameter profiles in phospholipid bilayers: computation from molecular dynamics simulations and comparison with 2H NMR experiments, European Biophysics Journal 36, 919 (2007).
- Landau and Lifshits [2007] L. D. Landau and E. M. Lifshits, Course of Theoretical Physics. Statistical Physics, Vol. 5 (Pergamon Press., Oxford [u.a.], 2007).
- Salem [1962] L. Salem, Attractive Forces between Long Saturated Chains at Short Distances, The Journal of Chemical Physics 37, 2100 (1962).
- Pabst et al. [2000a] G. Pabst, M. Rappolt, H. Amenitsch, S. Bernstorff, and P. Laggner, X-ray Kinematography of Temperature-Jump Relaxation Probes the Elastic Properties of Fluid Bilayers, Langmuir 16, 8994 (2000a).
- Pabst et al. [2000b] G. Pabst, M. Rappolt, H. Amenitsch, and P. Laggner, Structural information from multilamellar liposomes at full hydration: Full q-range fitting with high quality x-ray data, Physical Review E 62, 4000 (2000b).